Solid–State Hydrogen Storage Materials with Excellent Selective Hydrogen Adsorption in the Presence of Alkanes, Oxygen, and Carbon Dioxide by Atomic Layer Amorphous Al2O3 Encapsulation
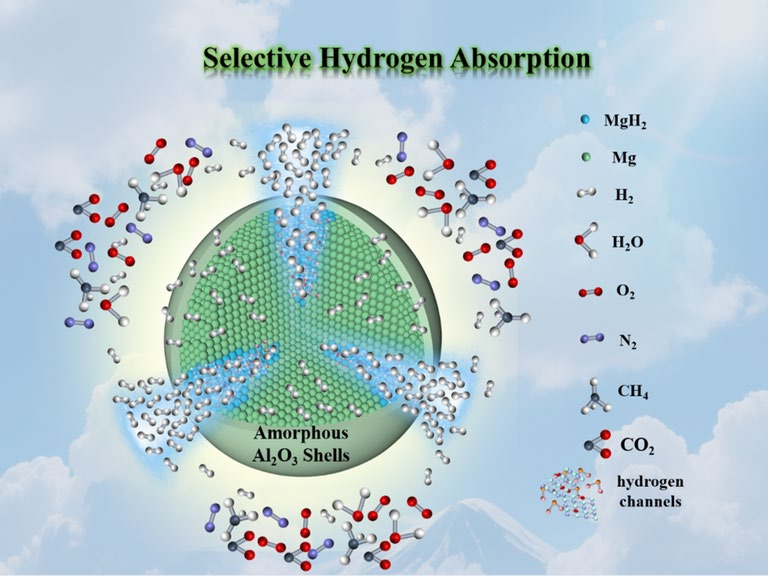
As hydrogen becomes a cornerstone of future clean-energy systems, practical solid-state storage must tolerate real, impure gas streams while retaining high capacity and fast kinetics. A research team led by Zhenyu Wang and Jinying Zhang (Xi’an Jiaotong University) reports a simple, effective solution: conformal, atomic-layer deposited amorphous Al2O3 shells (≈10 nm) on catalytically tuned MgH2–ZrTi particles (MgH2–ZrTi@Al2O3), which selectively admit H2 but block common contaminants (CH4, O2, N2, CO2) and deliver robust cycling and air stability.
Why Al2O3 Encapsulation Matters
- Selective adsorption: The amorphous Al2O3 shell permits rapid H2 permeation while preventing penetration or reaction of larger/more reactive species (CH4, O2, CO2), enabling hydrogenation from impure gas mixtures.
- Air stability: Coated particles show no detectable MgO or Mg(OH)2 after extended air exposure (months), a dramatic improvement over uncoated MgH2.
- Kinetics & capacity balance: The 10 nm shell preserves fast kinetics and high usable capacity by working in concert with internal Zr/Ti catalysts and hydrogen channels.
- Mechanical/chemical robustness: Amorphous shells remain intact after multiple de/re-hydrogenation cycles, supporting long-term operation.
Innovative Design and Features
- Atomic-layer engineering: Ultrathin, conformal Al2O3 layers were grown by ALD directly on MgH2–ZrTi particles, producing amorphous shells that are invisible in XRD but clear in TEM/elemental mapping.
- Synergistic core composition: MgH2 is modified with dispersed ZrO2 and few-layer Ti3C2 to create internal hydrogen channels and lower dehydrogenation temperatures (~185 °C onset for optimized composites).
- Mechanistic confirmation: MD simulations and experimental gas-uptake studies show H2 uniquely permeates the Al2O3 layer while other gases are adsorbed or shallowly intercalated, explaining observed selectivity.
- Operating window: Selective hydrogen uptake is achieved at moderate temperatures (75–125 °C), making solar-thermal or low-grade-heat charging feasible.
Key Performance Highlights
- ~4.79 wt% H2absorbed at 75 °C within 3 h under 10% CH4 + 90% H2; ~4.0 wt% absorbed at 100 °C in O2/CO2-containing mixes.
- Excellent cycling: ≈96.9% capacity retention after 30 cycles under impure H2; ~95.0% retention after 50 full de/re-hydrogenation cycles.
- No detectable oxidation products after months of air exposure for 10 nm coatings, while uncoated materials rapidly degrade.
Applications and Future Outlook
This ALD-encapsulation strategy opens a pathway to practical storage that accepts industrial/by-product hydrogen streams (e.g., coke-oven gas) without costly purification. Future work should explore scalable ALD workflows, alternative amorphous shell chemistries, and integration with system-level heat management for solar-assisted charging. This study presents a materials-level breakthrough toward deployable, selective, and air-tolerant hydrogen storage.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in