Some microalgae smartly switch energy systems to support growth under nutrient limitation
Published in Microbiology

Microbial rhodopsins are membrane proteins that convert light into biological signals or energy. They are an abundant family of photoreceptor proteins comprising a large diversity and representatives from all domains of life and even viruses. They largely function as light-driven ion pumps or channels, but also contain sensory-active and enzyme-sustaining variants. Nowadays, new rhodopsins are described on a regular basis and the explosion of research on microbial rhodopsin over the last decade has in large part been driven by the key role these proteins play in optogenetics, a methodology in neuroscience to study neural circuits and brain function.
Yet, optogenetics was still in its infancies and the explosion of research on microbial rhodopsins was still ahead when I arrived in Norwich, UK in fall 2008 to start my PhD in the new Lab of Thomas Mock at University of East Anglia to develop reverse genetic approaches in diatoms focusing on the polar model diatom Fragilariopsis cylindrus (Fig. 1; Otte et al. 2023).
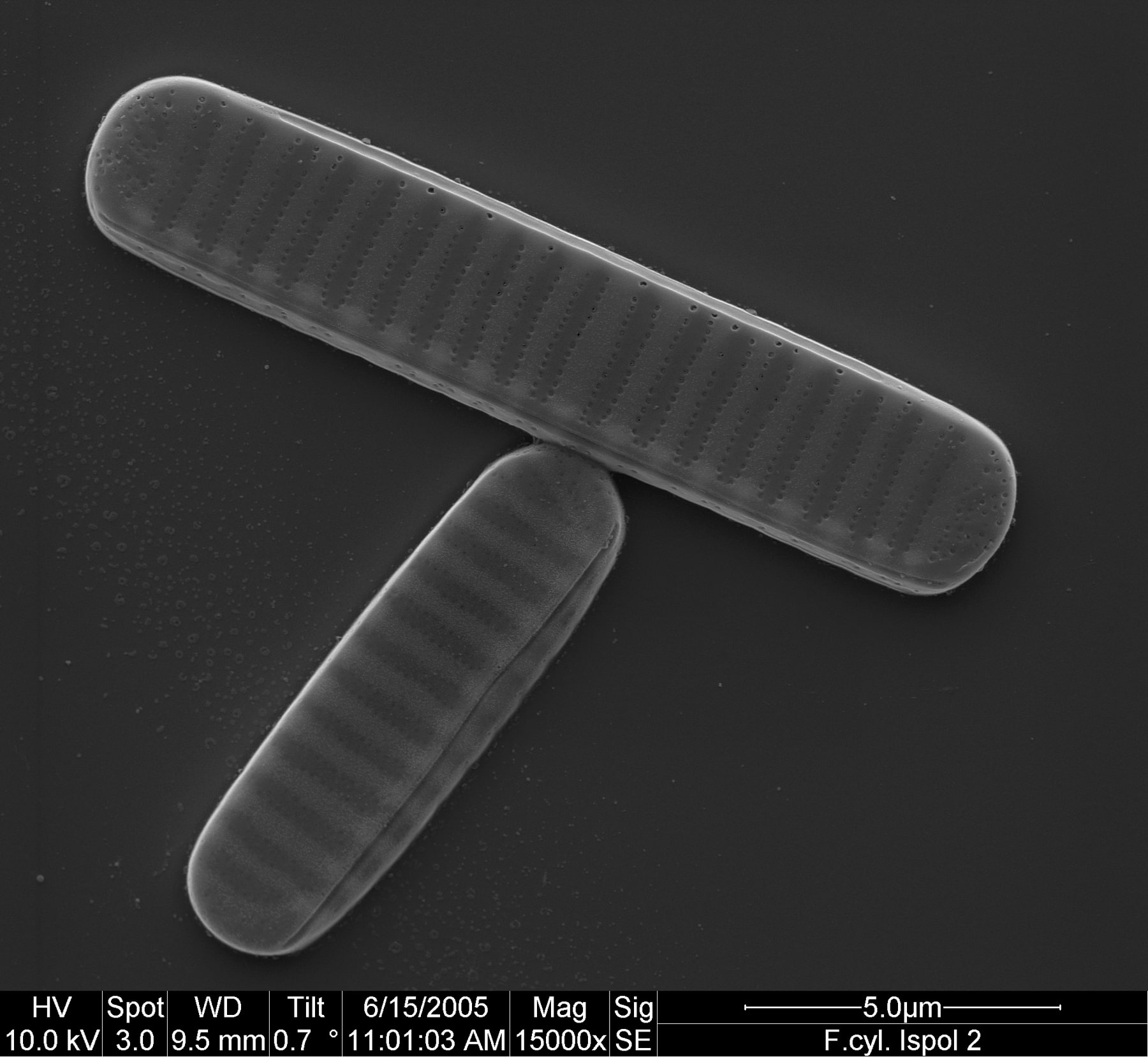
Photo: AWI/ Henrik Lange & Friedel Hinz, CC BY 4.0
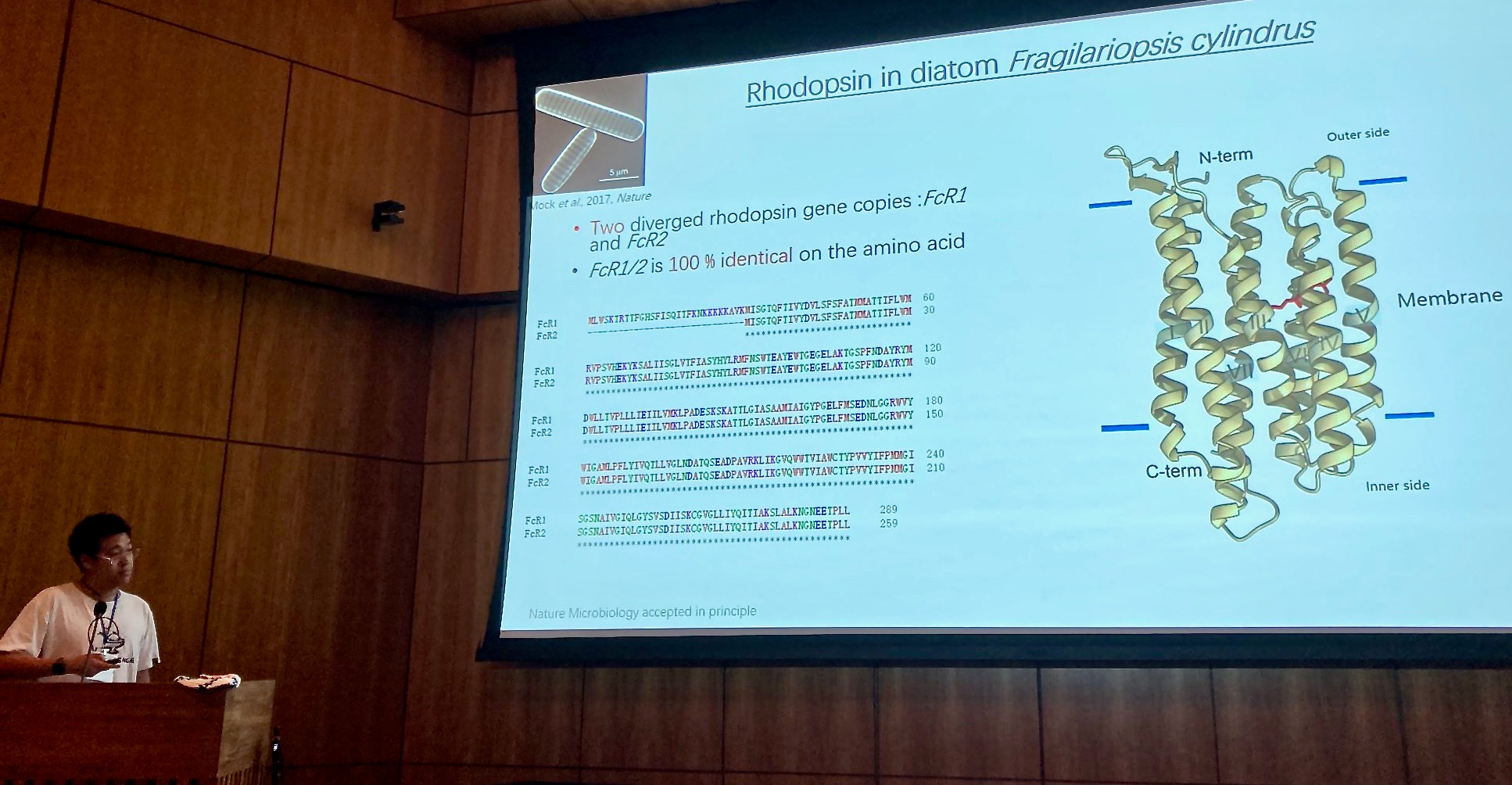
At that time the first draft genome sequence of Fragilariopsis cylindrus was just about to be released by the US Joint Genome Institute (https://mycocosm.jgi.doe.gov/Fracy1/Fracy1.home.html). When first screening the genome sequence in 2007, we were very excited to find genes that were not present in other diatom genomes that had been sequenced to that date (see 'Behind the paper' post at https://go.nature.com/3x7PDkk). One of them was a bacteriorhodopsin-like gene (Fig. 2), which had never been identified before in a marine photosynthetic microalga. This discovery marked the beginning of a more than decade-long and tortuous path of experiments and analyses including several international collaborations, to characterize the function and significance of this protein for marine phytoplankton.
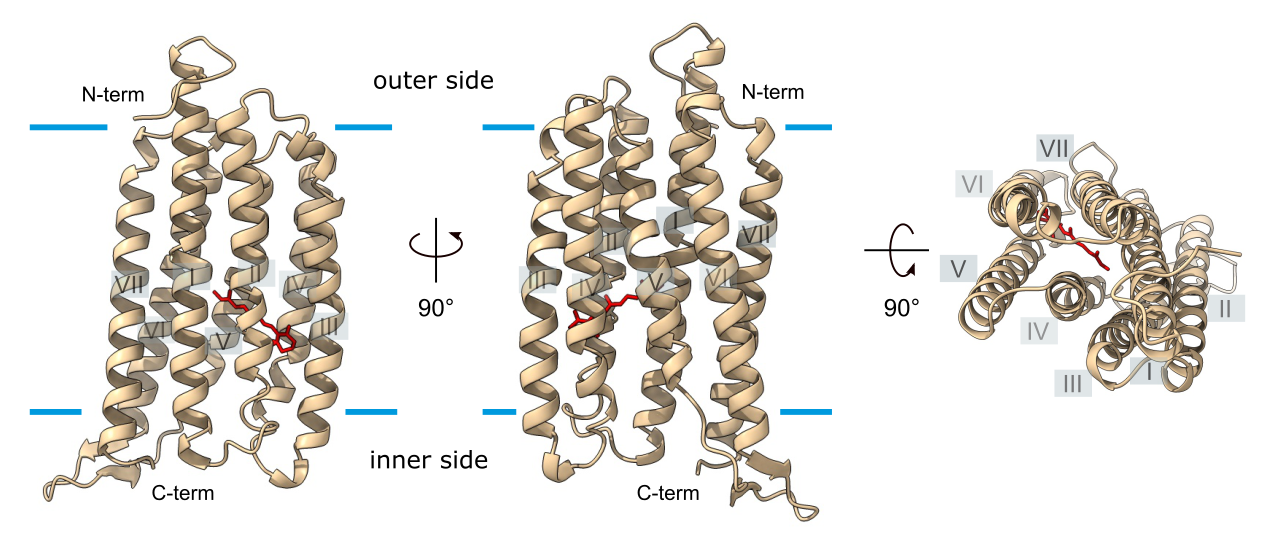
Figure: Strauss et al. 2023, CC BY 4.0
At the time of our discovery of a bacteriorhodopsin-like gene in F. cylindrus, it had been hypothesized, based on theoretical assumptions, that proton pumping rhodopsins provide an advantage for photosynthetic organisms during iron deficiency when chlorophyll-based photosynthesis that strongly depends on iron would be limited (Raven 2009). Our experimental strategy was thus designed to critically test this hypothesis using a combination of complementary approaches from cell biology to sequence analyses on a global level.
First, we used computational analysis and predictions and identified all residues and structural features responsible for ion transport as expected for a proton pump. Yet, sole presence of conserved residues of ion-transporting rhodopsins is not sufficient to prove functioning as proton pumps, because such residues are also present in some sensory rhodopsins. Even until today, only few microbial rhodopsins of eukaryotic microalgae have been experimentally characterized and we therefore designed a biophysical study to characterize the protein. Experimental characterization of transport proteins using heterologous expression in oocytes of the African clawed frog Xenopus laevis (Fig. 3) combined with voltage clamp techniques belongs to the methods of choice, allowing characterization of proteins from a broad range of eukaryotic organism. It even had already been successfully applied to identify and functionally characterize the diatom silicon transporter family back then (Hildebrandt et al. 1997).

oocytes and two-electrode voltage clamp assays were used
for biophysical characterization of the Fragilariopsis rhodopsin.
Photo: Brian Gratwicke, CC BY 2.0, via Wikimedia Commons
I therefore emailed an expert in rhodopsin research, Professor Georg Nagel at the Julius-Maximilians-Universität of Würzburg in Germany, asking if his lab would collaborate. I am still glad that he was hooked instantly, and my colleague Shiqiang Gao who was just about to start his PhD to characterize new photoreceptors to expand the optogenetic toolbox (Gao, S., PhD thesis, University of Würzburg (2015)) was keen to help (Fig. 4). Unfortunately, it proved to be very difficult to express the protein in Xenopus oocytes and the work was very frustrating over more than two years. Luckily, he never gave up and was persistent enough to find a way to increase the expression dramatically developing a protein engineering strategy that can be successfully transferred to other eukaryotic rhodopsins.
Photo: Shiqiang Gao, CC BY 4.0
During the same time, we were not sitting around in the other labs twiddling our thumbs. By further manual curation of the F. cylindrus genome, we identified an additional gene copy variant that shared complete amino acid sequence identity but containing a 30 amino acid N-terminal extension but had not been predicted by the automated genome annotation pipeline. We determined the specific expression of rhodopsin gene variants in F. cylindrus developing a variant-specific RT-qPCR assay. Strikingly, both variants showed a more than two orders of magnitude higher absolute gene expression in iron-limited F. cylindrus and one variant, FcR1, was only expressed during iron-limited growth. We also expressed Fragilariopsis rhodopsin and green fluorescence protein gene fusion constructs in model diatoms to determine their subcellular localization in the plastid and performed phenotyping experiments with F. cylindrus at green and red light to test for a potential advantage conferred by a putative ion-pumping rhodopsin performing best at green light (Fig. 5).

experimental set-up of diatom phenotyping experiments under green and red light (right).
Photos: Jan Strauss, CC BY 4.0
It was then Shiqiang (Fig. 4), who managed to amplify and clone the full-length gene of the FcR1 variant using complementary DNA (cDNA) as a template which I had extracted from iron-limited F. cylindrus cell cultures. Working with this variant, he first succeeded to achieve high expression levels in oocytes and to biophysically characterize the protein. I still remember reading his email that he had cloned the FcR1 variant and managed to measure pump signals in oocytes - it has remained my personal eureka moment!
During the years while all this work took place, more and more putative proton-pumping rhodopsins have been identified in marine eukaryotic microorganisms, including all major algal groups, where they have sometimes erroneously been attributed to the related bacterial proteorhodopsins. Yet, even if proven to function as proton pumps, the physiological benefit they bring to the organism remained notional at most. We therefore tried hard to get reverse genetics experiments with diatoms to work that would allow us to directly test if the Fragilariopsis rhodopsin can support growth when photosynthesis is iron limited.
Building upon all our experiments and experiences (including the most frustrating and failed ones!), like the finding that protein tagging of the diatom rhodopsin abolishes its proton pump activity, it was my colleague Longji Deng (Fig. 6), representing a new PhD student generation in the Mock Lab who succeeded to generate a rhodopsin knock-in gain-of-function mutant cell line of a non-rhodopsin-containing model diatom. Indeed, phenotyping assays of this rhodopsin knock-in mutant cell line showed significantly higher growth rates in comparison to its wild-type control under iron deficiency.
Finally, to evaluate the importance of such proton-pumping xanthorhodopsins in supporting algal growth in iron-limited surface oceans, we analyzed the abundance of their gene transcripts within the natural environment by analyzing 82 metatranscriptomes, environmental gene expression samples of eukaryotic microbes that had been sampled from the upper ocean and ranged from pole to pole (Martin, Schmidt et al. 2021; 'Behind the paper' post at https://go.nature.com/3AbyuFQ).
While both working in the Lab of Professor Alexandra Z. Worden at the GEOMAR Helmholtz Centre for Ocean Research Kiel in Germany, my colleague, Charles Bachy (now Station biologique de Roscoff, France), and I used phylogenetic placement of metatranscriptome sequences onto a custom rhodopsin reference tree to identify ~3,500 diatom rhodopsin transcripts. Advanced statistics using multiple regression analysis showed a positive correlation of the diatom rhodopsin transcript abundance and physiological iron stress.
Putting all these pieces of data together we were able to publish our paper last week (Strauss et al. 2023) after a long journey that I embarked on almost exactly 15 years ago as a young PhD student at the University of East Anglia. Looking at old pictures from me back then (see former members section of Mock lab website), when I was mostly working in the lab (Fig. 5) and today working as a Data Scientist at the German Maritime Centre, where I mostly turn coffee into code, may bear witness to the sweat, blood and energy it took to complete this paper. It required working at more than three different research institutes across two different countries including the University of East Anglia, the European Molecular Biology Laboratory (EMBL) and GEOMAR, obtain funding from different sources such as the National Environment Research Council (NERC) and EMBL Interdisciplinary Postdoc (EIPOD) programme and, not least, the endless enthusiasm, support, patience and persistence of my co-authors, my wife and my family to see this through.

Taken together, our study suggests that diatom rhodopsins play a particularly important role in the Southern Ocean (Fig. 7) which is chronically depleted in dissolved iron. Alongside previously identified strategies of modifications to the relative abundance of photosystem I and II and replacement of iron-containing ferredoxin and cytochrome c6 with metal-free flavodoxin and copper-containing plastocyanin, xanthorhodopsins provide another evolutionary strategy to support growth under iron-limiting conditions.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in