Sparkling electrolyte generate fuels
Published in Chemistry

The story of this paper starts from early 2020 when my family just moved to Montpellier. The sparkling water maker in the supermarket is on sale and we brought one without hesitation because I am addicted to drinking sparkling water. This sparkling water maker brings us some coolness on the hot summer days of south France (Now I miss the sunny day of Montpellier so much...).

Then it comes to the experiment, as all the CO2RR researchers, especially guys who work with H-cell know. Before each experiment, there is an essential but time-consuming process to saturate the electrolyte with CO2 by bubbling for around 30 mins. The idea comes from once I was preparing sparkling water by pushing a button 3 or 5 times and the CO2-saturated water is ready in 10 seconds. Why do not people use sparkling water makers to saturate the electrolyte to save some time? I tried it, and indeed, the saturation process was significantly reduced. However, it seems that the reaction was performed in a CO2 "super satureaturated" electrolyte and even the production of 2-propanol (a very rare product for CO2RR which has only been reported by a few papers) was detected by further NMR analysis. So definitely we found something very interesting and different from what we expected.
.jpg)
According to Henry’s law, under constant temperature, the amount of a given gas that dissolves in a given volume of liquid is directly proportional to the partial pressure of gas in equilibrium with the liquid. So we measured the CO2RR performance under elevated applied pressures and introduced a new CO2 supersaturation strategy for the direct conversion of CO2-to-C3 products. Performing the electro-reduction of CO2 under supersaturation conditions orients the reaction pathway towards the formation of 2-propanol. The mechanism responsible for the high selectivity towards the formation of C3 products was explored in detail using operando Raman, FTIR and hXAS spectroscopy together with ex-situ isotopic labelling experiments and DFT calculations. These results led to the identification of the role of *CO for C1-C1 coupling and *OCH2CH3 as a key intermediate for the selective formation of 2-propanol. Our experimental and calculation results highlight the importance of the CuAg alloy catalyst on the formation of 2-propanol and emphasize the advantage of the supersaturation strategy to increase the local CO2 concentration during the CO2RR, which improves the formation rate of *CO intermediates.
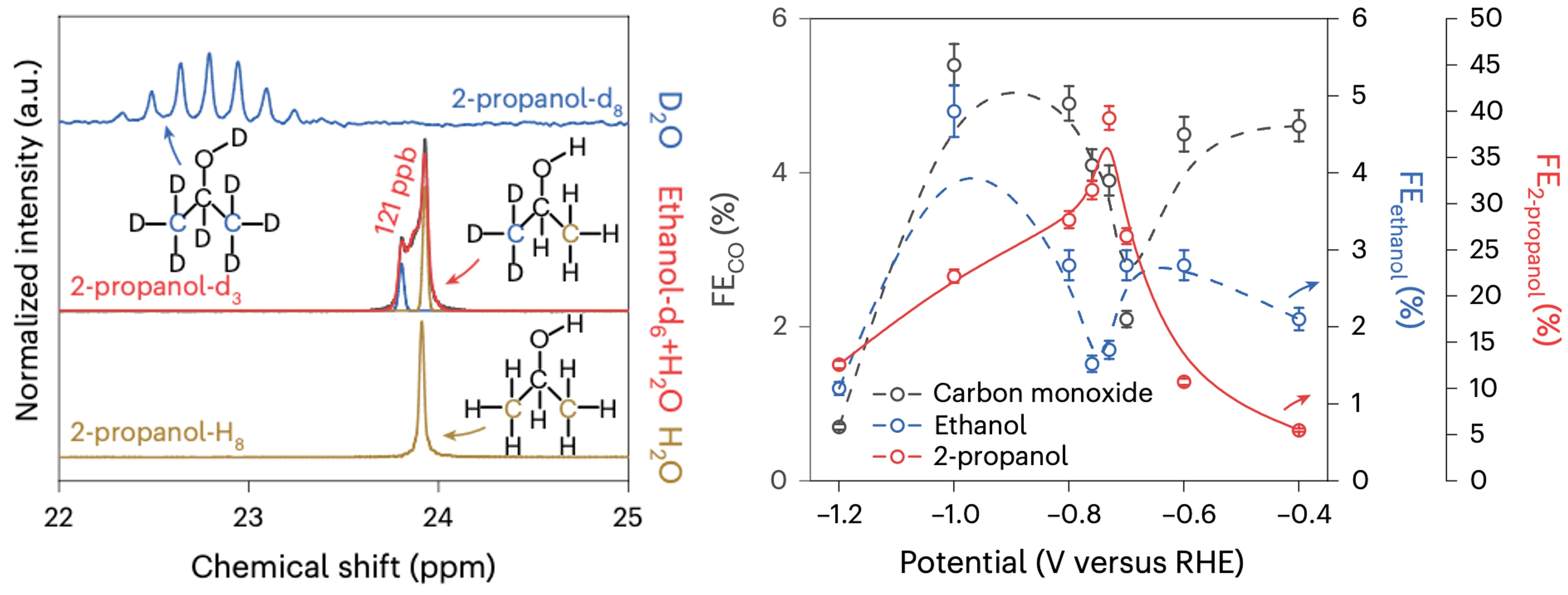
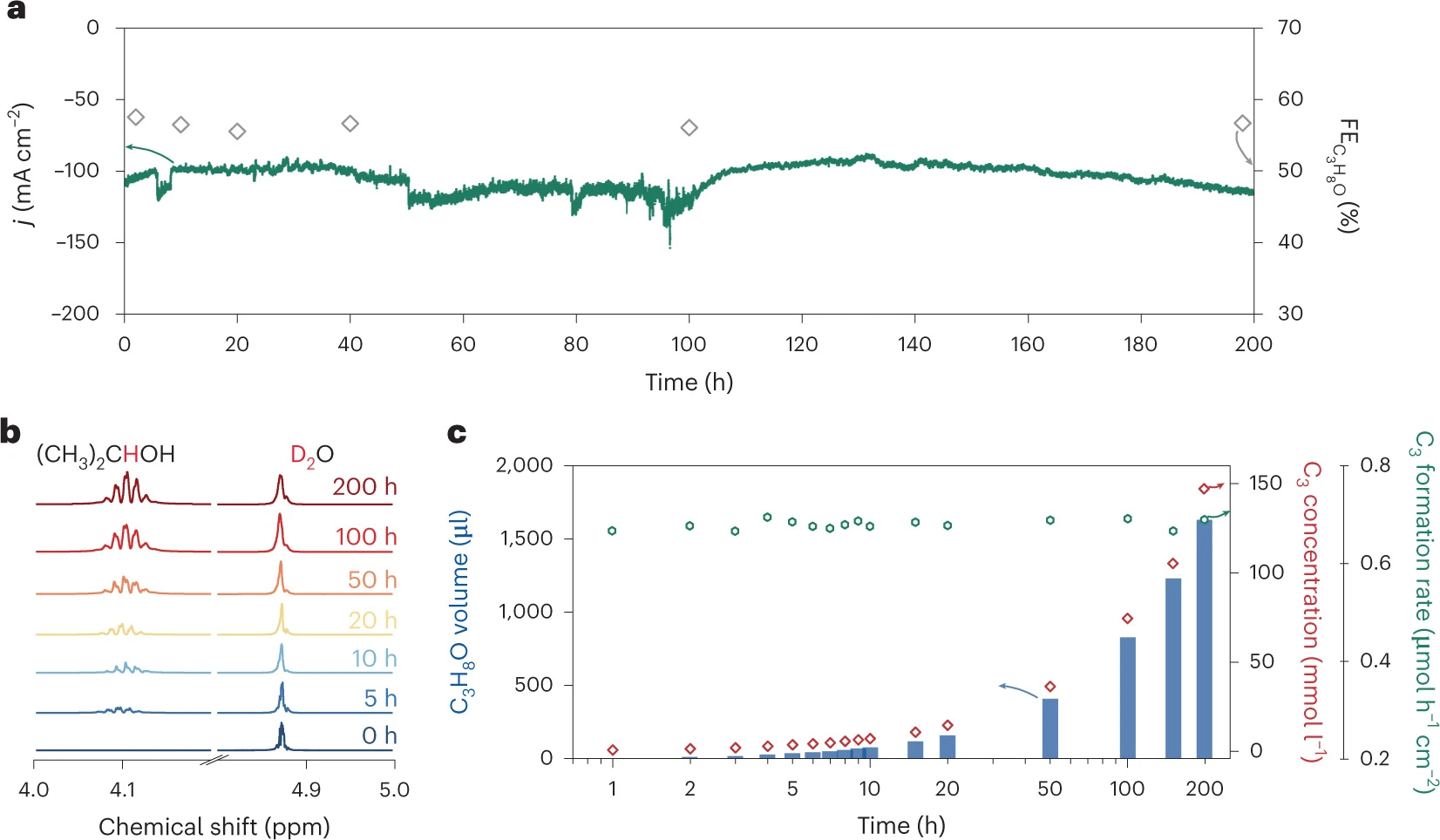
Finally, we achieved a record-high selectivity with the Faradaic efficiency for 2-propanol of 56.7 %, a specific current density of 59.3 mA cm-2, and operating stability for 200 hours at 10 bar (optimized CO2 pressure and concentration). The investigations provide the opportunity for fine-tuning the reaction pathway by controlling the local CO2 concentration and the formation rate of the catalytic favourable intermediates. We believe that the supersaturation strategy holds promise for applications in large-scale carbon fixation, the production of high-market value, energy-dense multicarbon molecules and the storage of energy in the form of chemical fuels.
This work cannot be done without the valuable collaborative efforts of Dr. Damien Voiry, Dr. Yang Zhang, Dr. Nicolas Onofrio, Dr. Eddy Petit (Institut Européen des Membranes), Prof. Xiaoqiang Cui (Jilin University), Dr. Luc Lajaunie (Universidad de Cádiz) and Dr. Jingyuan Ma (Shanghai Institute of Applied Physics, CAS).
Dedicated to my beloved Dr. Yang Zhang & Xiyao Qi (lovely Nuan Nuan).
You can read more about the study in Nature Catalysis.
Find me on my Twitter @Atome_Kun and @VoiryGroup to discuss this paper and more works on novel metallic materials and renewable energy conversion.
Some social media reports for this paper can be found through the following links:
Our work has been selected as the cover art for the April issue of Nature Catalysis! Super CO2!
Nature Catalysis 2023 Volumes 6 Issues 4

Reference:
1. Qi. K. et al. Unlocking direct CO2 electrolysis to C3 products via electrolyte supersaturation. Nature Catalysis. 10.1038/s41929-023-00938-z.
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in