Stem cells in organogenesis and regeneration
Published in Cell & Molecular Biology
https://link.springer.com/article/10.1186/s13287-025-04889-z
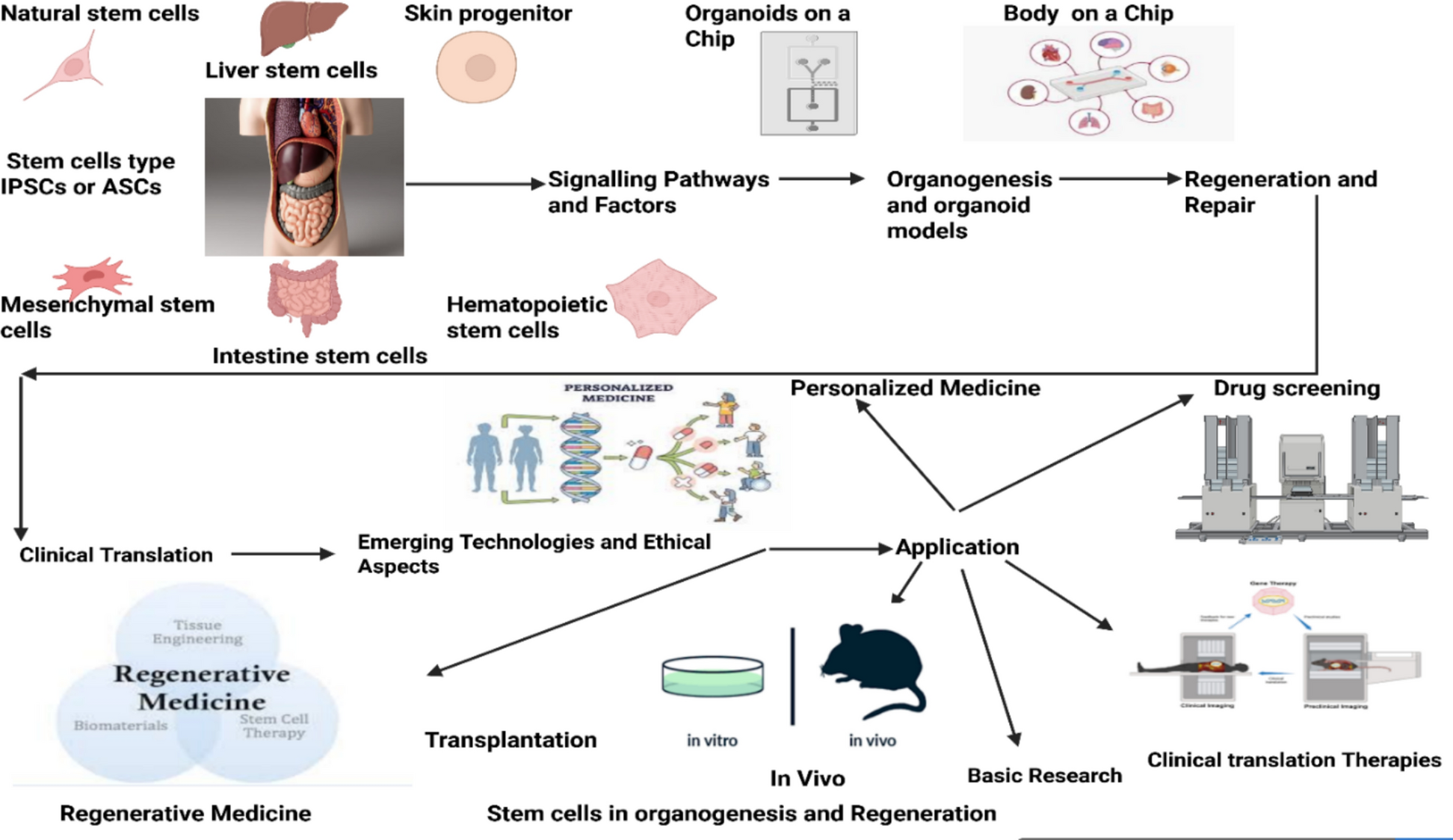
This review summarizes the fundamental role of stem cells in organogenesis, tissue maintenance, and regeneration. It outlines major stem cell types and their sources, emphasizing key signaling pathways—Wnt, Notch, Hedgehog, and BMP—that govern stem cell fate and lineage specification. The roles of embryonic stem cells, induced pluripotent stem cells, and mesenchymal stem cells in organ formation and tissue repair are discussed, along with advances in directed differentiation. The review highlights organoids as powerful models for studying development and disease, examines stem cell niches and microenvironmental regulation, and reviews translational progress, clinical applications, challenges, emerging technologies, and ethical considerations in stem-cell-based therapies.
Follow the Topic
-
Stem Cell Research & Therapy

The major forum for translational research into stem cell therapies. An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Neural Stem Cell-derived EVs for Treating Neurological and Neurodegenerative Disorders
As we advance our understanding of the role of neural stem cells (NSCs) and the composition of extracellular vesicles (EVs) they release, we begin to unveil promising pathways to address the challenges posed by debilitating neurological and neurodegenerative disorders. Recent advancements employing NSC-EVs in animal models of disease are showing promise for neuroregeneration and neurorepair. These breakthroughs involve the modulation of neuroinflammation, alleviation of amyloid-beta and phosphorylated tau-related pathological changes, repair of the blood-brain barrier, and improvements in cognitive and mood function. As we further explore these developments, the potential for enhanced synaptic plasticity and improved patient outcomes is becoming increasingly viable. NSC-derived EVs are laden with a rich cargo of microRNAs, proteins, and lipids, capable of mediating powerful antioxidant and anti-inflammatory effects, enhancing cognitive function, and providing neuroprotection. Moreover, the innovative engineering of NSC-EVs can significantly improve their therapeutic potential, positioning them as an ideal biologic for slowing down brain aging and combating a range of diseases, including the difficult-to-treat conditions such as Alzheimer’s disease, Parkinson’s disease, stroke, subarachnoid hemorrhage, traumatic brain injury, multiple sclerosis, and spinal cord injury. These disorders urgently call for groundbreaking therapeutic strategies, and harnessing NSC-derived EVs may pave the way for transformative interventions in the field of neurology.
We invite researchers to contribute to this special Collection by submitting original research articles. A few insightful review articles will also be considered.
Topics of interest include, but are not limited to:
- Mechanisms of NSC-EVs-mediated neuroregeneration and neurorepair
- The role of microRNA and protein cargo of NSC-EVs in neuroprotection
- Strategies for BBB penetration of intravenously administered NSC-EVs
- Targeting of NSC-EVs into specific cell types in the brain
- Mechanisms of antioxidant and antiinflammatory effects of NSC-EVs in the brain
- Modulation of synaptic plasticity by NSC-EVs
- Insights from studies of NSC-EVs in animal models of brain aging, Alzheimer’s and Parkinson’s diseases, traumatic brain and spinal cord injuries, and multiple sclerosis
- Pathway for clinical applications of NSC-EVs in neurological and neurodegenerative disorders
- Production of clinical-grade NSC-EVs
- Innovations in cell-free therapeutic approaches
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jun 05, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in