Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature
Published in Chemistry

Methane conversion to value-added molecules is one of the Holy Grails of modern chemistry. Methane is one of the most widespread molecules on the Earth. The vast and diverse feedstocks of methane vary from natural and shale gas to biogas and cow’s emissions. The key challenge in methane activation is the unique chemical stability of C-H bond, which results in both thermodynamic and kinetic limitations in methane conversion to valuable products [1]. The state-of-the-art methane chemical conversion technologies are energy-consuming and require very high temperatures (>800 °C). They often suffer from poor selectivity and insufficient stability due to the catalyst deactivation and major sources of carbon dioxide emissions.
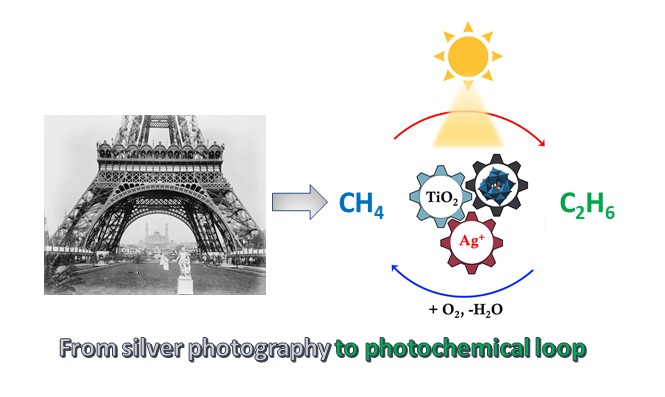
Solar light provides a formidable opportunity to activate methane at ambient temperature. In our group (see website: www.suscat.org), we have started working on the photocatalytic methane activation in 2016 with the arrival of Xiang Yu as a PhD student. In addition to being an excellent PhD student, Xiang Yu is a passionate photographer. We have performed numerous experiments with methane oxidation over TiO2 supported photocatalysts with deposited metals at room temperature. Very soon, we uncovered that the presence of tungsophosphoric (HPW) heteropolyacid with Zn leads to highly intensive formation of CO via intermediate formation of methyl carbonate [2].
As continuation of this research, we tested silver containing heteropolyacid over titania in the anaerobic transformation of methane. Surprisingly, extremely selective synthesis of ethane in the amounts almost exactly corresponding to the silver content was observed under irradiation at room temperature. The material has turned black, because of the transformation of cationic Ag to metallic silver. The chemical reactions resemble those occur in silver photography, which transforms light-sensitive silver salts into metallic silver in the presence of light (Figure). In our process, cationic Ag in the presence of light activates methane at ambient temperature with generation of CH3 methyl radicals and protons. Recombination of the radical species yields higher hydrocarbons.
In order to reach quantitative conversion of methane to higher hydrocarbons under irradiation, we proposed with Dr Vitaly Ordomsky and other coauthors a concept of photochemical loop, which involves separation of the reaction and regeneration stages. In the reaction stage, methane under irradiation is stoichiometrically coupled to ethane with sliver being simultaneously reduced to the metallic state. In the regeneration stage, light re-oxidizes under air metallic silver back to the cationic state. The proposed cycling between cationic and metallic states with selective methane coupling can be reproducibly repeated over and over again over the developed photosensitive nanocomposites.
Now we are focusing on deeper understanding of mechanism of methane activation, development of new materials able to activate methane in visible light and design of self-regenerating photochemical systems.
Link to the manuscirpt: https://doi.org/10.1038/s41560-020-0616-7
Reference
[1] Tang P, Zhu Q, Wu Z, Ma D. Methane activation: the past and future. Energy. Environ. Sci. 7, 2580-2591 (2014).
[2] Yu, X., De Waele, V., Lofberg, A., Ordomsky, V. and Khodakov, A.Y. Selective photocatalytic conversion of methane into carbon monoxide over zinc-heteropolyacidtitania nanocomposites, Nature Commun. 10, 700 (2019).
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in