Surface (S-) layer proteins are a bridge that connects multispecies microorganisms in anammox biofilms
Published in Microbiology
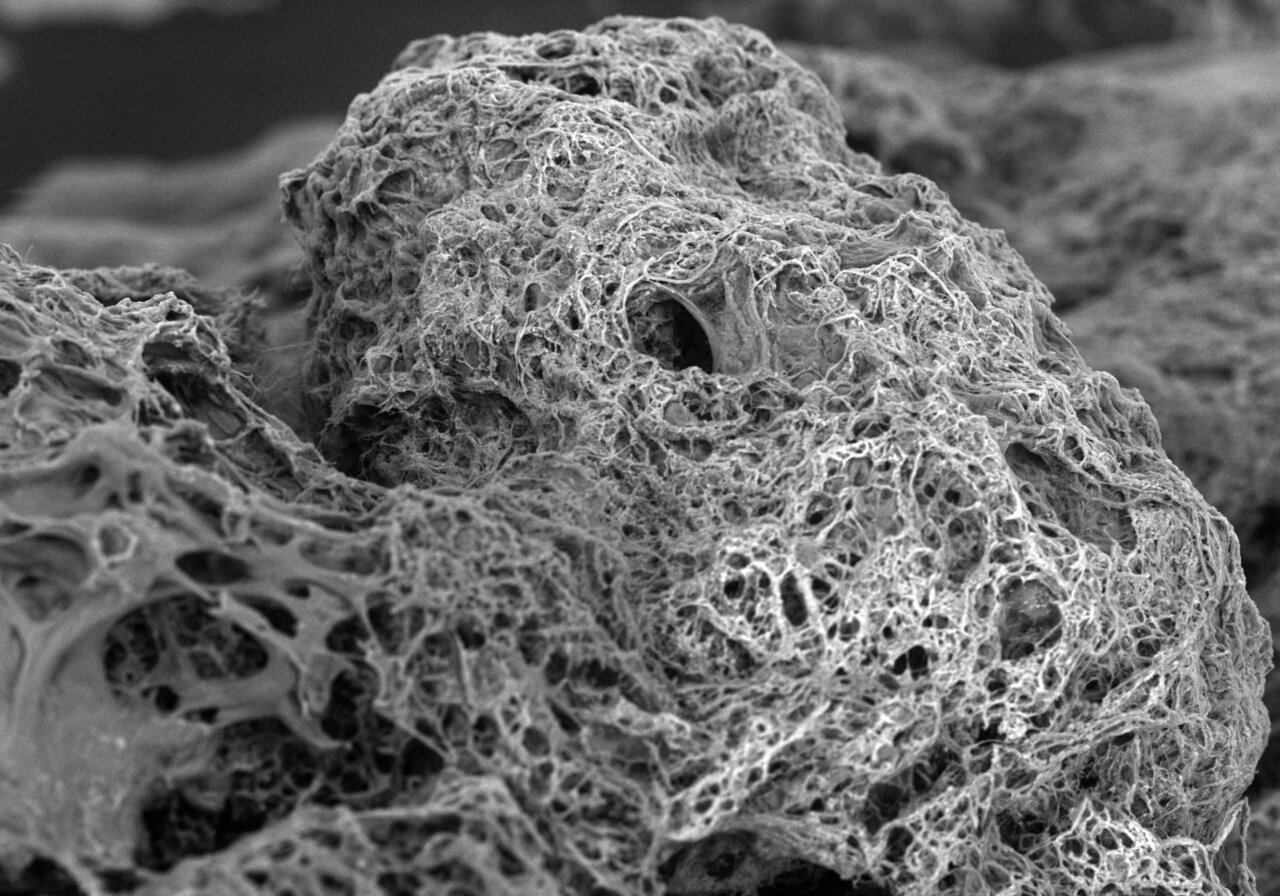
Biofilms are a collection of bacteria, existing mostly as communities of multiple species housed in a slimy extracellular polymeric matrix. Biofilms are everywhere and contribute greatly to the biotransformation of key nutrients, as seen in wastewater treatment processes. But the matrix also provides biofilm-embedded bacteria with a robust scaffold for residing in, imparting them with emergent properties such as stress resistance, e.g., enabling persistent microbial infections. The scaffold also serves to bring microorganisms together, enabling cooperation for resource cycling and sharing, and hence promote optimal fitness for all members of the community.
The role that each bacterium performs in metabolic partnerships have been the subject of much research, and can be inferred from studies of their genetic code. In contrast, the roles of EPS polymers have been vastly understudied. A particular shortcoming involves understanding how the microbes are constructing the matrix to create a scaffold for optimal community cooperation, and how this ultimately affects community function.
All bacteria in the biofilm potentially benefit from this shared infrastructure, which raises the question of whether they all contribute to its assembly, or whether this service is provided by specific bacterial species. In the case of the latter, it is pertinent to understand how the bacteria achieve this outcome and the extent to which conspecifics are taxed for this service?
To address these questions, we used a model anaerobic ammonium oxidation (anammox) biofilm community. Anammox is an important industrial bioprocess that contributes greatly to the efficient N-removal/recycling from wastewater worldwide. Anammox biofilms appear as small red cauliflower-like granules comprising a community of different microorganisms held together by an EPS matrix. This microbial lifestyle provides a good example of cooperation to achieve complex biotransformations. To date, a major difficulty in unravelling such biological systems involves understanding the protection imparted by the matrix and the organisation of cells within. The matrix EPS are complex, poorly characterised and sparsely described in online databases (e.g., NCBI). These EPS are structurally and compositionally diverse and, by nature, are often insoluble in conventional solvents.
To enhance our understanding of such complex microbial systems, our first step was to extract, isolate and identify the major exopolymer from anammox biofilm using a novel ionic liquid-based solvent, previously described by our lab (1). This was identified as a putative surface (S-) layer protein expressed by a key member of the anammox consortium. This was unforeseen as S-layer proteins are not commonly recognised as exopolymers. Using immunostaining and fluorescence in-situ hybridisation analysis, we found that the S-layer protein was localised on the exterior of anammox cells, as expected from a surface protein. However, it was also present in high abundance at the edge of the biofilm where no anammox bacteria were located.
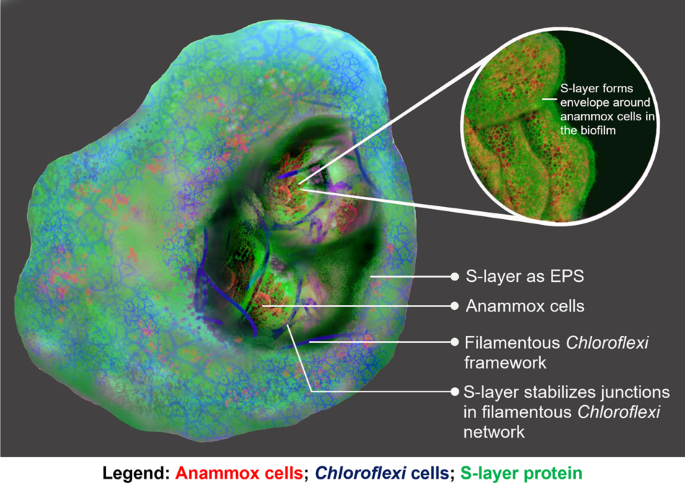
Moreover, filamentous bacteria belonging to the genus Chloroflexi were seen to form crosslinked networks at the edge of the biofilm as well as throughout the biofilm interior, where it accommodated and demarcated the active S-layer protein-expressing anammox cell clusters. Further microscopy analysis revealed that the S-layer protein was particularly abundant at the Chloroflexi network junctions. One possible explanation for this arrangement is that the anammox expressed S-layer protein can assist another species to assemble into key biofilm structures. Further, our experimental findings also suggested that the S-layer protein is likely acting as the nutrient source for Chloroflexi bacteria. This therefore suggests that anammox and Chloroflexi cooperate in biofilm matrix assembly, whereby the anammox S-layer protein is a ‘common good’ exopolymer that establishes the biofilm scaffold, and in turn facilitates diverse mutualistic metabolic relationships that ultimately underpin the anammox bioprocess.
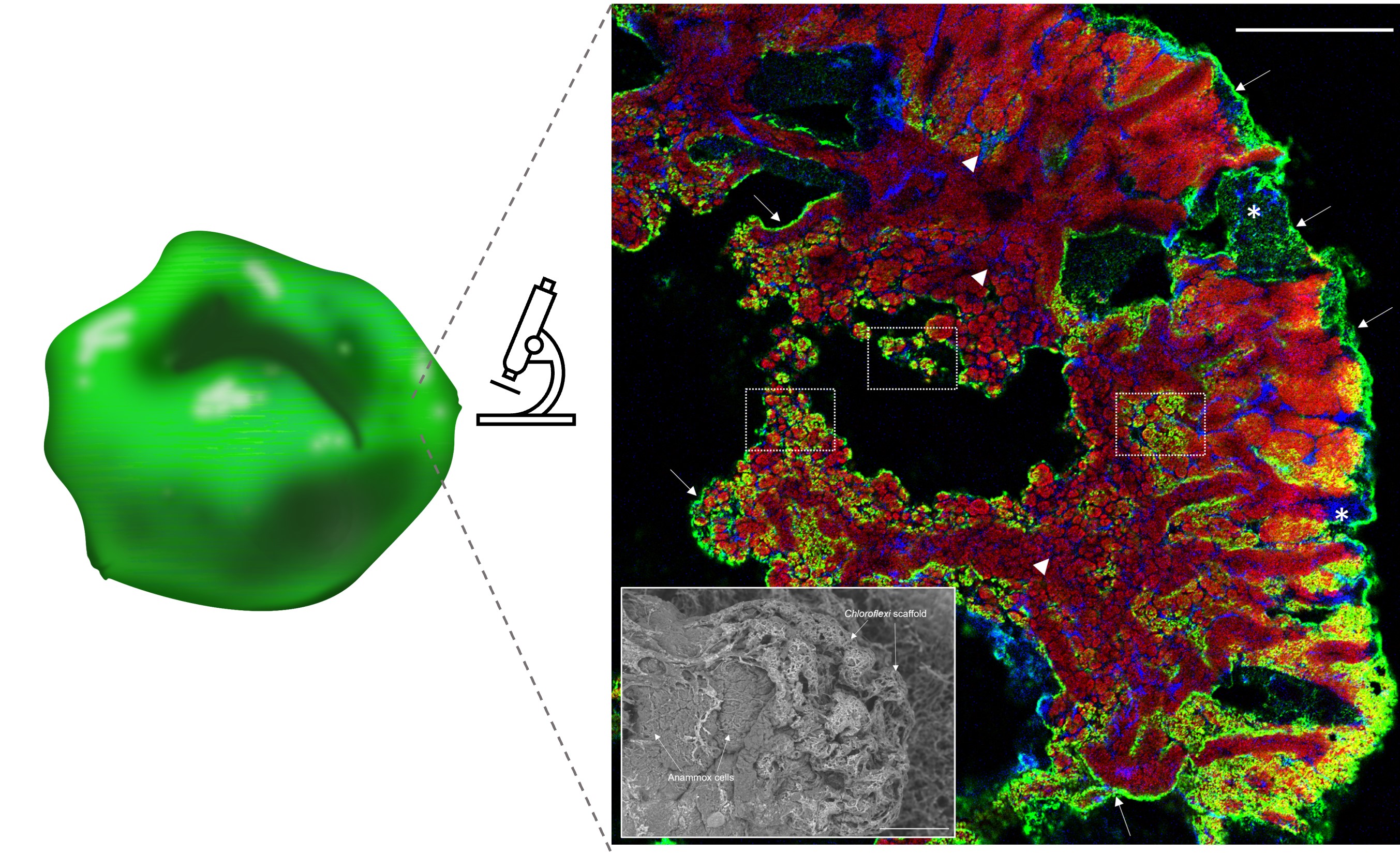
Anammox granule with S-layer protein (green) located on the cell surface (left). Confocal laser scanning micrograph (right) shows an anammox biofilm thin section with Chloroflexi (blue) demarcating anammox cell clusters (red) at the internal channels between anammox cells (arrowheads) and at the periphery of the biofilm (asterisk). The distribution of S-layer protein (green) on anammox cell cluster surfaces (white dotted box) and at the biofilm edges (white arrows) is overlaid. Scale bars indicate 20 µm. Inset: Scanning electron micrograph shows the cross-linked Chloroflexi network surrounding anammox cells of anammox granular sludge. Scale bar indicates 50 µm.
This study demonstrates that S-layer proteins are not only surface proteins, but also serve as key matrix components and ‘public good’ biofilm matrix building materials. While this was observed in our model system, Chloroflexi is a common scaffold builder in activated sludge. The metabolic and structural cooperation between the biofilm community member is facilitated by matrix EPS, a common mechanism in wastewater treatment biofilms. Our study has elucidated the role of S-layer producing species in structuring functional biofilm consortia, based on a direct microscopic illustration of how microbes work together to build biofilm scaffolds, particularly to support complex biotransformations like anammox.
Reference
- Wong LL, Natarajan G, Boleij M, Thi SS, Winnerdy FR, Mugunthan S, et al. Extracellular protein isolation from the matrix of anammox biofilm using ionic liquid extraction. Appl Microbiol Biotechnol. 2020;104(8):3643-54.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in