Tackling gliomas with CAR-T cells
Published in Bioengineering & Biotechnology

Following closely on the successes of CD19-directed CAR T-cell therapy in the treatment of pediatric leukemia, researchers are quickly expanding the range of indications for these engineered immune cells. Because of the modular nature of CARs, replacing their specificity (defined by their antigen-binding domain) is a relatively simple process; considerably more challenging is finding appropriate tumor-associated antigens that are not abundant in healthy tissues (to avoid immunopathology), or making sure that the CAR T cells do not overreact and trigger dangerous cytokine storms in patients.
One of the tumor-associated antigens being explored for CAR T-cell therapy is the disialoganglioside GD2. This protein has a restricted expression pattern in healthy cells, but has been frequently found to be overexpressed in tumors of the nervous system, including neuroblastoma and glioma. These early observations prompted the development of CAR T cells specific for GD2, which have been tested in small clinical trials of patients with neuroblastoma1-3. Now, a team of researchers from the Stanford University School of Medicine show that GD2-specific CAR T cells effectively target and clear patient-derived glioma tumors transplanted into the central nervous system of mice4, suggesting that GD2-CAR T cells may be an alternative for these patients.
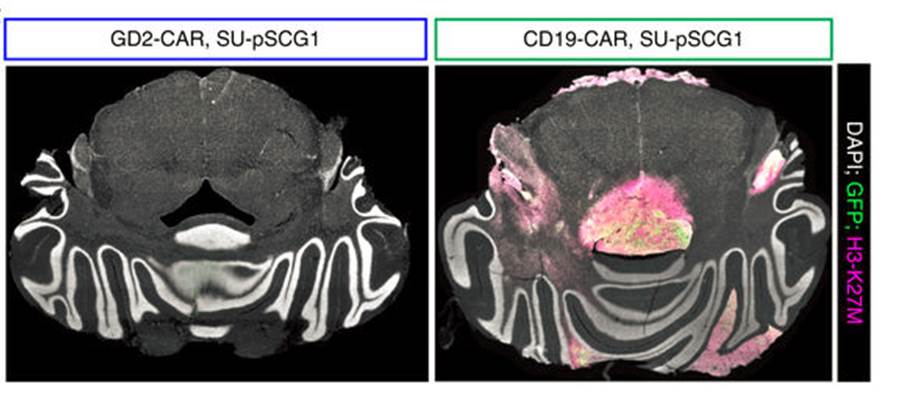
Figure 1. Immunofluorescence images of H3-K27M+ glioma cells implanted into the mouse central nervous system. Mice that received GD2-CAR T cells (left) had virtually undetected tumor cells, while tumors in mice that received a CAR T cells with an unrelated CAR (right) were free to expand. Adapted from Figure 4g (ref.4). Macmillan Publishers.
The researchers focused on a specific glioma type—diffuse intrinsic pontine glioma—that is associated with a K27M mutation in histone H3. Often aggressive and ‘universally fatal’, these tumors ‘diffuse’ along the brain stem, making surgical removal not an option. To better achieve a clinically relevant setup in their xenograft glioma mouse model, the researchers implanted patient-derived tumor cells in the spinal cord or the thalamus of the mice, and proceeded with infusion of the engineered CAR T cells 7–8 weeks after tumor establishment. In both situations the CAR T cells effectively ablated the vast majority of the tumor cells (Figure 1). However, mice with thalamic gliomas developed substantial toxicity, possibly due to local edema, highlighting the importance of the neuroanatomical location of this type of tumors when considering therapeutic strategies and monitoring for adverse effects.
References:
1. Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
2. Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
3. Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 25, 2214–2224 (2017).
4. Mount, C. W. et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat. Med. doi:10.1038/s41591-018-0006-x (2018).
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in