Taurolidine-Containing Solution to prevent Cardiac Implantable Electronic Device Infection
Published in Biomedical Research, General & Internal Medicine, and Surgery
Various cardiac arrhythmias necessitate the implantation of cardiac implantable electronic devices (CIEDs). However, this procedure is accompanied by inherent risks of adverse events (AEs). These AEs can be categorized into procedure-related complications, such as pneumothorax, vascular injury, and hematoma formation, as well as device-related issues, including lead dislodgement or malfunction. Among these complications, CIED-related infections (CIEDIs) are particularly concerning due to their potential to increase morbidity, mortality rates, and the utilization of healthcare resources. The substantial morbidity and mortality associated with CIEDIs, as documented in the medical literature, have led to the implementation of performance-enhancing measures. Surveillance systems aimed at monitoring hospital quality and patient satisfaction have been established across Western healthcare systems. Reported rates of CIEDIs are utilized to withhold reimbursement for affected providers in the US Medicare system. Consequently, significant efforts have been directed towards developing evidence-based guidelines for the prevention and treatment of CIEDIs. These guidelines systematically evaluate and implement strategies designed to reduce the incidence of CIEDIs globally.
The European Centre for Disease Control and Prevention (ECDC) focuses primarily on hospital-acquired infections, including CIED-related infections; however, reporting from contributing centers is inconsistent. Consequently, rates of CIEDIs exhibit considerable variability and may not be adequately monitored, leading to misunderstandings and underutilization of guideline-recommended treatments. Discrepancies in reported rates across various studies hinder accurate data interpretation, particularly regarding the true prevalence of CIEDIs. Research has documented a rise in CIEDI rates over recent decades. CIEDI is classified as a clinical diagnosis that requires laboratory tests and imaging procedures, such as transesophageal echocardiography, transthoracic echocardiography, and positron emission tomography using Fluorodeoxyglucose computed tomography (FDG-PET-CT). These diagnostic modalities are utilized to identify the CIED and its associated hardware, such as leads, as the source of infection. Major CIEDIs encompass any infection involving the surgical site, including localized generator pocket infections and lead-related infective endocarditis. This classification facilitates the definitive diagnosis and treatment of CIEDIs according to updated criteria.
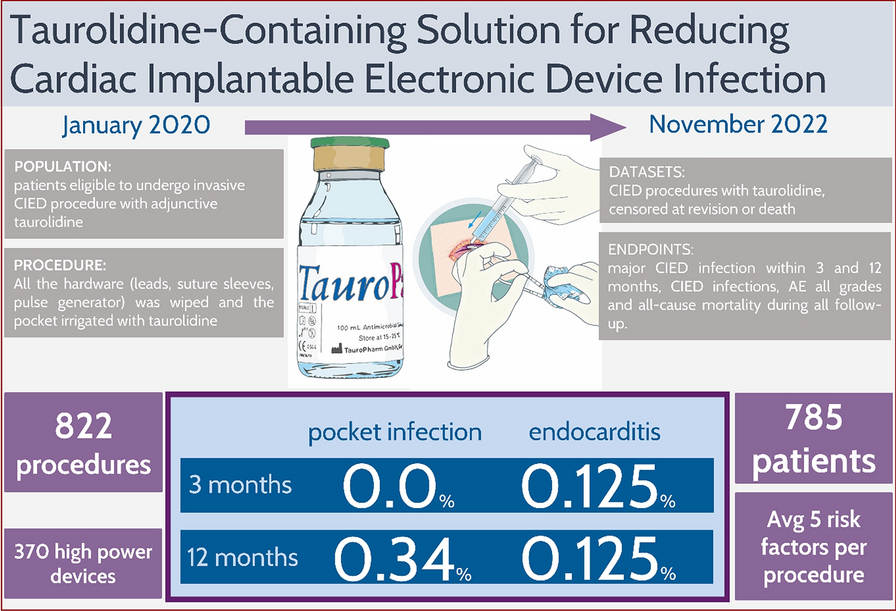
The recent milestone publication by Vonthein et al., part of the European TauroPace registry (ETPR), provides crucial insights into the efficacy of taurolidine in preventing cardiac implantable electronic device (CIED) infections, reinforcing its potential in this domain. The unique properties of taurolidine, as emphasised in the study, are particularly significant in addressing the persistent challenges posed by biofilms, which form rapidly once a conditioning film—inevitably developing on any inert material implanted in the human body—becomes contaminated. Biofilms, composed of hydrogel-enclosed bacterial microcolonies, communicate and organise sophisticated defence mechanisms that undermine the effectiveness of conventional antibiotic treatments, particularly in the acidic and anaerobic conditions found deep within the biofilm structure. The registry’s findings highlight taurolidine’s distinct mechanism of action, whereby it metabolises to release highly reactive particles that irreversibly bind to and destroy pathogens without requiring pathogenical metabolism. This further eliminates the risk of resistance development, a critical limitation of traditional antibiotics and even conventional antimicrobials. Taurolidine’s prolonged antimicrobial activity, as demonstrated in the registry, makes it an effective strategy for preventing biofilm-associated infections during CIED procedures. Moreover, its excellent biocompatibility further establishes taurolidine as a highly promising alternative to conventional therapies for the prevention of CIED infections.
The above mentioned milestone publication from the ETPR is part of an investigator-initiated clinical development plan aimed at establishing the adjunct use of Taurolidine in cardiac implantable electronic device procedures. This project follows the IDEAL framework, which outlines a systematic approach to clinical research across several stages. In the initial phase, known as the Idea (IDEAL Stage 1), the focus is on the common use of Taurolidine in locking solutions as recommended by existing guidelines. Taurolidine has demonstrated efficacy in various surgical settings, including lung transplantation, dental implantation, and spinal fusion surgery. Notably, it has been utilized as a last-resort treatment for serious infections related to ventricular assist devices and localized major CIED infections. During the Development phase (IDEAL Stage 2a), a standardized operating procedure for the routine use of adjunct Taurolidine during CIED placement was established by participating physicians. The ETPR will document the use of adjunct TP in a multicentric, prospective registry, aiming to follow 2,300 procedures until 2030. The registry will focus on interventions registered before procedures to accurately estimate infection rates, and the safety of adjunct TP use will be monitored and reported to enhance post-marketing surveillance. The Exploration phase (IDEAL Stage 2b) includes a study comparing major CIED infection rates with and without adjunct TP, providing preliminary data for future studies. A case-control study of CIED extractions will analyze the reasons for removal, matching controls with similar risk factors for CIED infections, which will generate sufficient data for multivariable modeling to inform participant selection for subsequent randomized controlled trials (RCTs). In the Assessment phase (IDEAL Stage 3), treatment guidelines may incorporate findings from a first RCT of Taurolidine against standard care. The design aims to optimize participant selection for maximum treatment benefit, with a primary endpoint established to demonstrate direct efficacy. Finally, in the Long-term phase (IDEAL Stage 4), the ETPR will accumulate extensive data over time to more precisely estimate small event rates and follow participants for long-term effects. Insights from the controlled cohort study, matched case-control study, and interim analyses will inform the planning and initiation of an eventual RCT, with the registry's continuation supporting ongoing surveillance of established practices in CIED procedures. Overall, the ETPR aims to provide comprehensive insights into the safety and efficacy of taurolidine as an adjunct treatment in CIED procedures, ultimately contributing to improved patient outcomes and informing future clinical guidelines.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in