The art of 3-D bioprinting for organ regeneration
Published in Bioengineering & Biotechnology

Having recently penned some ideas across epigenetic mutations, to epigenetic engineering, and DNA nanotechnology here. In this post, I similarly share my opinions on the dynamics of regenerative medicine for regenerative organoid and organ bioprinting. Bioengineers seek to develop biological replacements for dysfunctional organs or injured tissues via regenerative tissue engineering. Tissue engineering to restore intact organs in the lab, is a nontrivial task that depends on the ample availability of a source of human cells and a few other requisites, which typically include the following:
- Pluripotent stem cells or adult stem cells with self-renewing properties that present an unlimited cell source,
- A suitable matrix that can support the architecture of target tissue, and
- A capacity to maintain organ viability with integrated neural and vascular networks to bioengineer models of early organogenesis, and fully-form 3-D bioprinted organ constructs for implantation or drug screening efforts.
Human organs are intricate and highly complex structures formed via several functional tissue types to perform distinct functions [Cui 2017]. Bioprinting holds great promise to repair or replace organs that undergo dysfunction or failure due to traumatic injury or disease [Ong 2017].
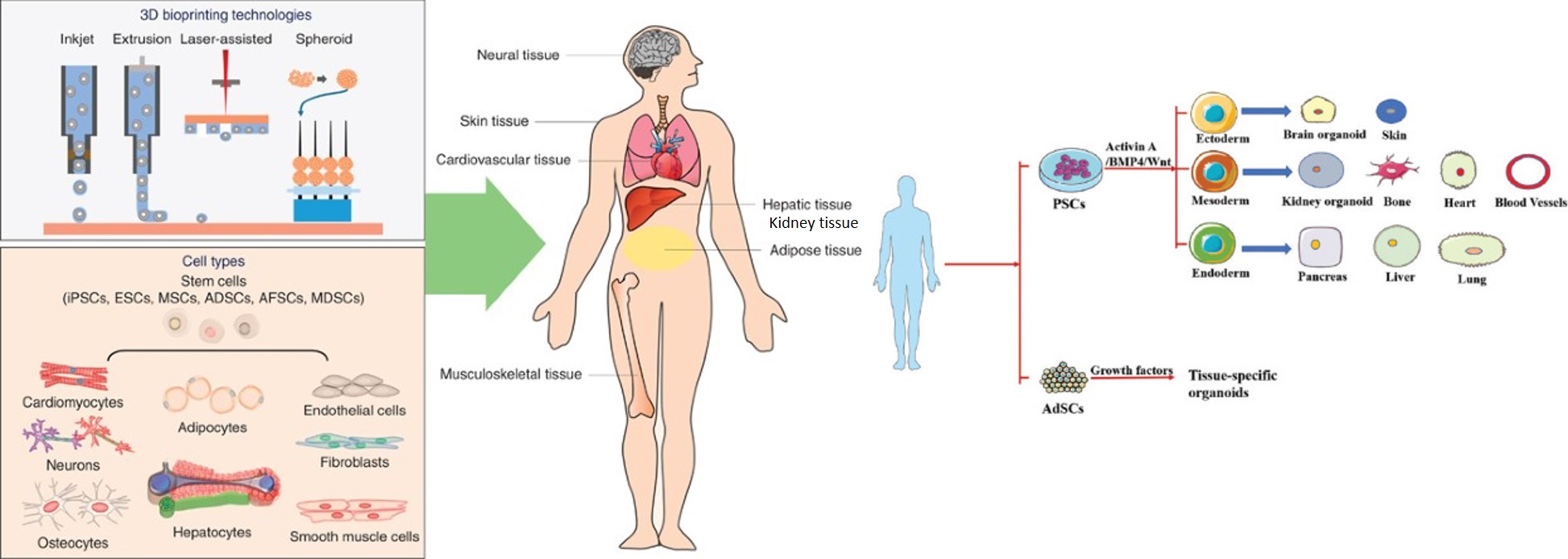
Figure 1: Bioprinting organs and organoids. Schematic illustration of the 3-D bioprinting technologies and the cell types used to 3-D bioprint diverse organ systems [Ong 2017]. Organoids are identified and developed with pluripotent stem cells or adult stem cells [Ren 2021].
Notable tissue engineering approaches are widely studied in cartilage, bone, kidney, and skin in an application-specific manner with sophisticated vascular and neural network integration [Agarwal 2020]. 3-D bioprinting is an automated additive process that offers a pioneering advantage to precisely position multiple cell types and bio-factors layer-by-layer, to generate multiscale architectures of biochemical and structural complexity [Ren 2021] (Figure 1). In this post, I briefly explore the process of tissue engineering with human pluripotent stem cells derived from progenitor cells to 3-D bioprint kidney and cardiovascular tissues/organoids/organs, in the lab with multiple functions.
The basics of 3-D bioprinting – bottom-up engineering an organ.
Bioengineers aim to innovate the strategies of tissue regeneration to facilitate organ repair and also form a theranostics tool to conduct drug screening, by first regenerating specific components of human organs in the lab. Notable advances include a focus on 3-D bioprinting stem-cell derived human organoids, with droplet-based, extrusion-based, and laser-based bioprinting methods to recapitulate the organ microenvironments through bottom-up engineering, to reduce the risk of immunological graft rejection [Zhang 2015] (Figure 2).

Figure 2: (a) a structured diagram of the various 3-D bioprinting methods, and (b) simplified illustrations of typical 3-D bioprinting techniques for tissue regeneration [Cui 2017].
To truly appreciate bioprinting, I retraced the steps of its origin to an offshoot of stereolithography – a liquid, photopolymer-based manufacturing method pioneered by Charles Hull in 1986, the invention subsequently set the tone to herald 3-D printing technology. Cellular bioprinting was a relatively recent invention based on traditional 2-D inkjet technology in 2003 [Wilson 2003], although one of the first commercial bioprinters were only developed quite recently in 2009 [Jose 2016]. The additive manufacturing capacity of 3-D bioprinting offers a platform for patient-specific treatment to bioengineer artificial implants or complex tissue constructs. While the basic method is mainly based on 2-D printing, it is defined by three key stages -
- pre-bioprinting to obtain preliminary medical images of the tissue/organ of interest,
- the actual bioprinting stage after bioink selection, and
- analyzing culture conditions post-bioprinting.
In its composition, bioinks typically contain biomaterials, bioactive components, and cells. The design of bioinks for 3-D bioprinting are listed on table 1 [Ren 2021]. These engineering techniques offer better options when compared to autografts or allografts to accomplish well-matched patient tissue repair [Goldberg 1987], [Ren 2021].
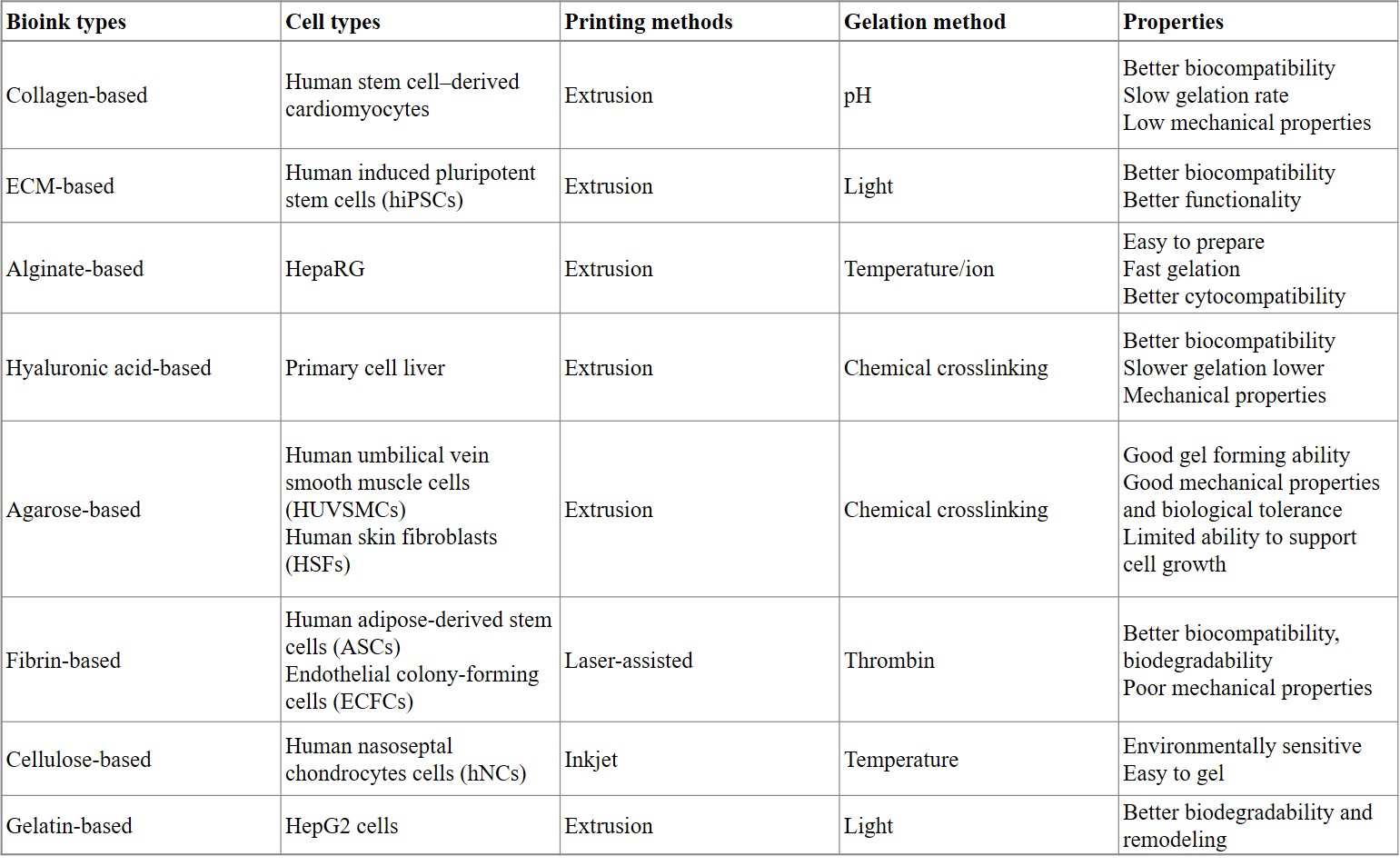
Table 1: Comparing the performance of different bioinks to form organoids [Ren 2021].
Bioprinting kidney organoids – a preview to organ physiology
Before recreating organ physiology in the lab, I am interested in understanding how such intercellular networks can retain long-term biocompatibility and cell viability for drug screening and other applications? How can we accurately recreate ordered tissue structures in a regenerative environment to ensure prolonged functionality in a translational capacity? How do we successfully integrate seamless vascular and neural networks into an organ generated in the lab? Does the development of organoids offer a preview to the process and functions? (yes).
To answer most of the questions – first, organoids are different from compact organ structures since they are tiny, self-organized 3-D tissue cultures derived from stem cells that can be crafted to replicate the complexity of a native organ. They can represent tissue regions of interest by recreating specific types of cell-to-cell networks ranging in size from less than a width of a hair, to a few millimeters. Organoids are composed of pluripotent stem cells or adult stem cells, the former pass through the endoderm, ectoderm, and mesoderm to differentiate into the phenotype of a required organ. Adult stem cells simply facilitate the segregation of growth-factor induced stem cell populations to create tissue-specific organoids (figure 1).
Kidney organoids can be manufactured by differentiating human pluripotent stem cells to form kidney constituents for drug screening, disease modeling, and tissue regeneration for renal replacement [Lawlor 2020]. Most efforts in the labs have relied on extrusion-based three-dimensional cellular bioprinting to create high throughput kidney organoids with a viable and reproducible cell number.

Figure 3: Anatomy of the kidney. A schematic representation of the anatomy of the kidney with details of the nephrons and glomerulus compartment where the filtration occurs, followed by A) recreating a renal proximal tubule in vitro model within a 3-D printed perfusable chip after manufacturing a hollow channel and encapsulating with a gelatin-fibrin extracellular matrix hydrogel. B) proximal tubule-like fully epithelialized region seeded with proximal tubule epithelial cells and C, D) control groups [Fransen 2021].
Two main groups are pivotal to bioengineer kidney models; extrusion or microfluidic-based, and droplet on-demand methods that can regulate the accuracy of cell morphology and ensure long-term cell viability [Cui 2017]. In terms of cellular architecture, the bioprinted kidney models are mainly based on epithelial and endothelial cells to emulate the proximal renal tubule and its interactions with the adjacent vasculature (figure 3) [Lawlor 2021], [Fransen 2021].
Peering into the details to see the bigger picture.
Since details literally make the bigger picture (to answer most questions); there is a possibility of depositing kidney organoids by using an automated extrusion-based 3-D cellular bioprinter, which includes Hamilton syringe carriers to facilitate the precise regulation of mechanical volume extrusion, tip positioning, movement, and speed. Using an automated script, the bioprinter can accurately deposit a specific volume of induced pluripotent stem cell paste at a defined needle tip location, to form bioprinted organoids with spontaneous nephron formation by 20 days of culture [Takasato 2016].
Recent work has highlighted the possibility of using 6-well or 96-well organoid bioprinting to devise a high throughput organoid platform for drug testing (Figure 4). The method can be customized to precisely biomanufacture uniformly patterned kidney tissue sheets with functional, proximal tubular segments [Lawlor 2021]. The automated extrusion-based bioprinting method for kidney organoid production can deliver high throughput organoids with quality control, scale, and structure to facilitate the applications of stem-cell derived, bioprinted human kidney tissue.
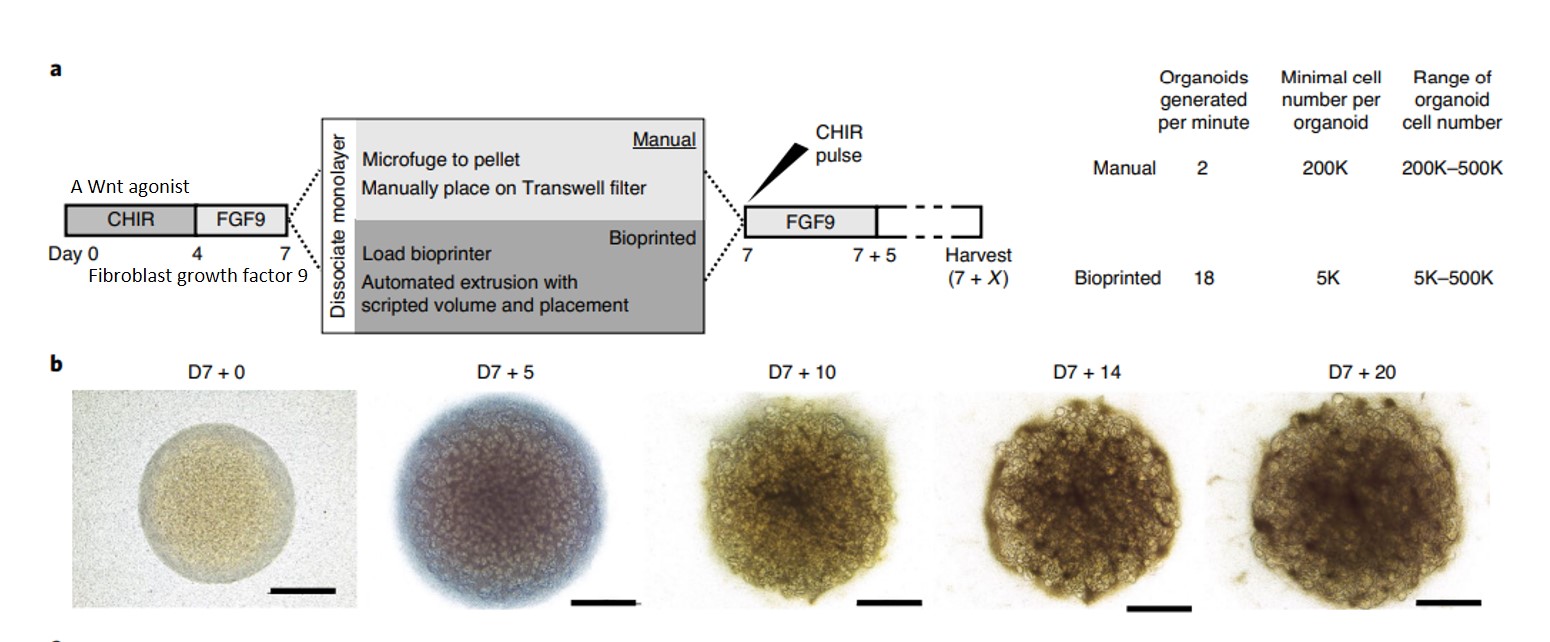
Figure 4: Generating highly reproducible induced pluripotent stem-cell derived kidney organoids via extrusion-based cellular bioprinting. A) an illustrated protocol comparing the manual versus bioprinted kidney organoid formation. B) bright field images of the micro-mass cultures from the first day of bioprinting to day 20 of culture to show spontaneous nephron formation [Lawlor 2021].
Such bioprinted organoids interestingly contain classically patterned nephrons, podocytes, proximal tubules, distal tubules/loop of Henle thick ascending limb and connecting segments of the ureteric epithelium as visualized via cell immunofluorescence. Further investigations with advanced microscopy can reveal bioprinted kidney organoids to have morphological equivalence to the manually developed kidney organoids [Lawlor 2021]. Experimentalists have shown the possibility of recreating renal tissue with similar functionality to its in vivo counterparts relative to glomerular filtration, tubular reabsorption, and water secretion. Such organoid patches can generate larger fields of functional kidney tissue for bioengineering and novel drug screening functions [Lawlor 2021]. A list of kidney compartments developed in the lab and their functionalities are listed on table 2.
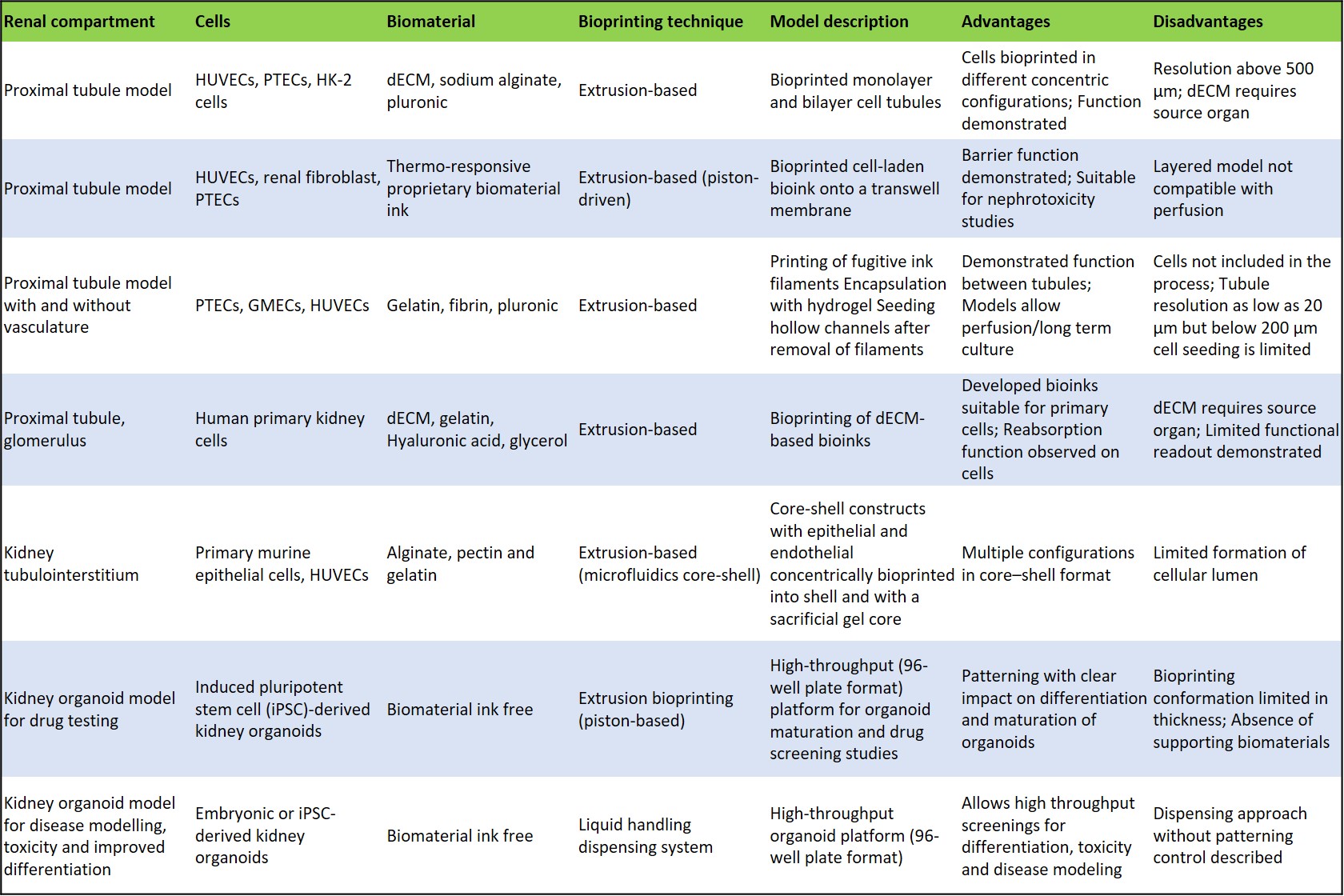
Table 2: Comparing the bioprinted renal models developed so far [Fransen 2021].
Bioprinting an organ in the lab – a recap and a classic example: the heart
Beyond organoids, the process of bioprinting an actual organ in the lab is comparatively more complex, as represented stepwise in figure 5. The method can be completed as a scaffold-based or scaffold-free process. In the scaffold-based method, a biomaterial matrix forms the initial 3-D layer for cellular deposition in the form of a hydrogel, nanofiber, or film onto which the bioink made of biomaterials or bioactive compounds can be patterned. The 3-D construct should closely mimic the native extracellular matrix environment for the cells to proliferate. The scaffold-free process allows direct printing of biomolecules and cells onto the substrate.
A variety of additive manufacturing methods have been developed to selectively pattern cells and biomaterials to bioengineer viable constructs through inkjet based bioprinting [Cui 2009], extrusion-based [Jones 2012], laser-assisted bioprinting [Keriquel 2017], and stereographic lithography based 3-D bioprinting [Dean 2012]. An alternative to 3-D bioprinting through regenerative medicine includes the possibility of generating a completely vascularized heart framework, by first enzymatically decellularizing a cadaveric heart, to provide an extracellular matrix that can be re-popularized in a bioreactor for cardiac organogenesis [Jung 2016].
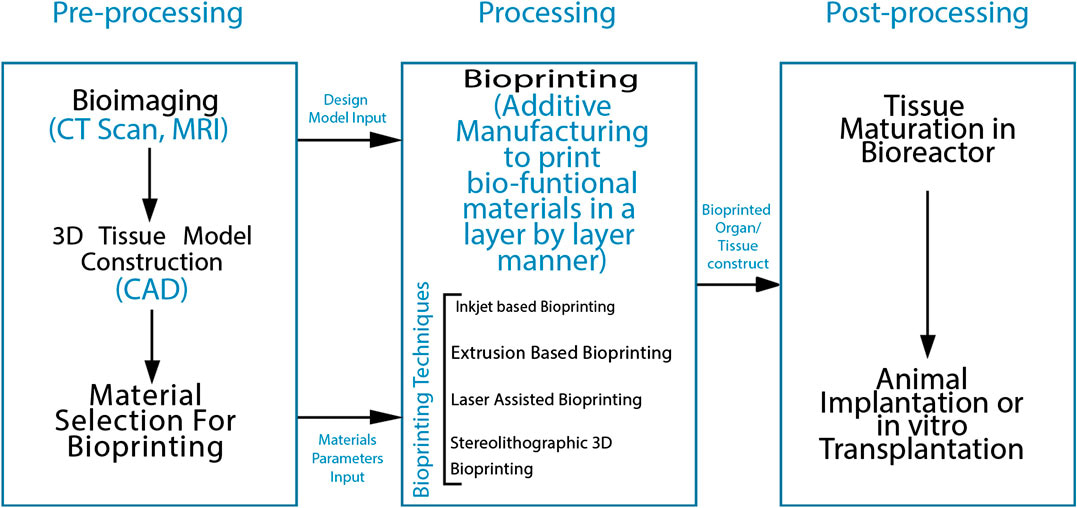
Figure 5: A flow diagram of the process of 3D Bioprinting [Agarwal 2020].
Building a heart one layer at a time
Recreating cardiac tissue in the lab is a classic example of 3-D bioprinting. Developing a functional cardiac construct to maintain the auto-rhythmic nature of the myocardium is challenging due to the complexity of the tissue, which requires the integration of multiple stem cell sources, including cardiomyocytes, fibroblasts, and endothelial cells [Vunjak-Novakovic 2010]. At first, a 3-D model can be developed through graphic modeling interphases based on clinical data obtained from clinical imaging such as MRI and CT-scan inputs. Using image segmentation processes, the patient-specific 3-D model can illustrate the desired or target cardiovascular tissue as a cue for anatomic geometry.
The bioengineered tissue constructs can undergo maturation in a bioreactor to customize its functionality to contract, and facilitate the delivery of blood, alongside mechanical and electrical stimulations to maintain the quintessential tissue morphology and function [Agarwal 2020]. Bioengineers hope to model regenerative tissues to treat heart disease and heart failure, conduct toxicological studies and implement personalized drug tests and enable personalized organ regeneration [Jung 2016]. For example, the recent potential to 3-D bioprint live replacement parts to repair the hearts of babies born with congenital heart disease is a transformational medical breakthrough that transcends science fiction [Kolesky 2016].
Multimaterial 3-D bioprinting can create thick human tissues with an engineered extracellular matrix, embedded vasculature, and multiple cell types (Movie 1) [Kolesky 2016]. As with all bioengineered organ structures or tissues, it is vital to maintain cell viability of bioinks for cardiac bioprinting. Such efforts are rewarded by using natural and synthetic polymers to form hydrogels gelatin, collagen, and hyaluronic acid in the bioink composition. Heart valve engineering with 3-D bioprinting is, however, rife with multiple design issues during the generation of functional and biocompatible valves, necessitating further studies to form more complex adult cardiomyocytes and valve interstitial cells [Bajaj 2014].

The promise of regenerative medicine
From the perspective of materials science and bioengineering, both bioprinting and organoid bioengineering are promising concepts in the field of regenerative medicine. In this post I ask and answer some key themes involving bottom-up engineering that offer insight into the field of experimental medicine. The methods emphasize the reconstruction of tissue structures to rebuild physiology with form and function, with scope to generate personalized 3-D printed organs that meet diverse, patient-specific functional requirements, to facilitate personalized medicine and precision diagnostics. While organoid bioprinting is still in its infancy, the combined methods can allow the realization of diverse organs of interest soon. Even though the process of post-printing maturation is still limited for 3-D bioprinting, the bio-fabricated in vitro models are instrumental to investigate drug toxicity and mimic pathophysiology to understand disease mechanisms and provide a unique platform to test new drugs.
Header Image: Building a vascular network using embedded 3D printing. The fluorescent material is later removed to create a channel network that can be connected to a pump for feeding tissues. Credit: Stanford Medicine, Lucile Packard Foundation.
References:
- Cui H. et al. 3D Bioprinting for Organ Regeneration, Advanced Healthcare Materials, doi: 10.1002/adhm.201601118
- Ong C. et al. 3D bioprinting using stem cells, Nature Pediatric Research, doi: https://doi.org/10.1038/pr.2017.252
- Ren Y et al. Developments and Opportunities for 3D Bioprinted Organoids, International Journal of Bioprinting, doi: 10.18063/ijb.v7i3.364
- Agarwal S. et al. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration–A Review, Frontiers in Mechanical Engineering, doi: https://doi.org/10.3389/fmech.2020.589171
- Zhang X. et al. Tissue Engineering Applications of Three-Dimensional Bioprinting, Cell Biochemistry and Biophysics, doi: 1007/s12013-015-0531-x
- Wilson W. et al. Cell and organ printing 1: protein and cell printers, Wiley, doi: 10.1002/ar.a.10057
- Jose R. et al. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting, ACS Publications, doi: https://doi.org/10.1021/acsbiomaterials.6b00088
- Goldberg V. et al. Natural history of autografts and allografts, Clinical orthopeadics and related research, doi: PMID: 3315383
- Lawlor K. et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation, Nature Materials, doi: https://doi.org/10.1038/s41563-020-00853-9
- Fransen M. et al. Bioprinting of kidney in vitro models: cells, biomaterials, and manufacturing techniques, Essays in Biochemistry, doi: 10.1042/EBC20200158
- Cui H. et al. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs via Dual 3D Bioprinting: Integrating Regional Bioactive Factors into Architectural Design, Advanced Healthcare Materials, doi: https://doi.org/10.1002/adhm.201600505
- Jones N. et al. Science in three dimensions: The print revolution, Nature, doi: https://doi.org/10.1038/487022a
- Keriquel V. et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications, Scientific Reports, doi: https://doi.org/10.1038/s41598-017-01914-x
- Dean D. et al. Continuous digital light processing (cDLP): Highly accurate additive manufacturing of tissue engineered bone scaffolds, Virtual and Physical Prototyping, doi: https://doi.org/10.1080/17452759.2012.673152
- Jung J et al. Solid organ fabrication: comparison of decellularization to 3D bioprinting, Biomaterials Research, doi: https://doi.org/10.1186/s40824-016-0074-2
- Vunjak-Novakovic, G. et al. Challenges in Cardiac Tissue Engineering, Tissue Engineering Part B: Reviews, doi: https://www.liebertpub.com/doi/10.1089/ten.teb.2009.0352
- Kolesky D. et al. Three-dimensional bioprinting of thick vascularized tissue, Proceedings of the National Academy of Sciences of the United States of America, doi: https://doi.org/10.1073/pnas.1521342113
- Bajaj P. et al. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annual Review of Biomedical Engineering, doi: https://doi.org/10.1146/annurev-bioeng-071813-10515




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in