The best of both worlds for Candida albicans: pan-azole antifungal resistance can be cost-free
Published in Microbiology

Background
Fungal infections, despite their widespread occurrence, are the most overlooked infectious diseases. In 2022, the World Health Organization (WHO) estimated that less than 1.5% of infectious disease research funding goes to studying fungal infections1. This disparity has also skewed antimicrobial resistance research toward antibiotics and antivirals, leaving antifungals understudied2.
Consequently, the number of antifungal resistance mutations reported in the literature remains relatively small compared to the large number of potential resistance mutations. For example, Candida albicans is the most common cause of fungal infections, and azoles are the most widely used class of antifungals. Yet, when I started this project, I found less than 20 experimentally validated resistance mutations in Erg11, the enzyme targeted by azoles in fungi. Furthermore, these resistance mutations were rarely tested across multiple azole drugs. Thus, although resistance to more than one azole drug had been observed, it remained unclear whether this cross-resistance was the rule or the exception. In addition, most resistance mutations were not assessed in the absence of drugs, leaving the question of whether or not they carried a fitness cost unanswered. In the few cases where fitness was assessed without a drug, no cost was observed, but this may have been due to a sampling bias.
In the Landry Lab, one of our goals is to develop resources and tools to detect, predict and fight antifungal resistance, and to do this we need reliable data. Therefore, one of the motivations behind this work was to generate the most comprehensive catalog of experimentally validated Candida albicans Erg11 azole resistance mutations. This includes not only mutations that were observed in patients but also those likely to confer resistance in the future. With this dataset, we also wanted to address concerns about azole cross-resistance and to determine whether Erg11 resistance mutations carry a fitness cost.
Thousands of Candida albicans Erg11 mutants were assessed
One of the challenges of studying fungal pathogens is the difficulty of genetically manipulating them. Although methods have been developed, engineering large numbers of mutants in fungal pathogens remains a challenge. Our solution to this problem was to use our favorite model organism, the yeast Saccharomyces cerevisiae. We genetically engineered S. cerevisiae to study Erg11 of fungal pathogens by heterologous expression from a plasmid.
Next, we constructed (presumably) the largest set of Erg11 mutations to date, with nearly 4,000 mutants of C. albicans Erg11 expressed in S. cerevisiae. We then competed all the variants in parallel in the presence of six different azoles to measure resistance to each individual azole as well as cross-resistance. To assess the fitness cost of the mutations, we also competed the variants in the absence of azoles. We used high-throughput sequencing at the beginning and at the end of the competitions to determine the frequencies of the variants and infer which were resistant (Figure 1).
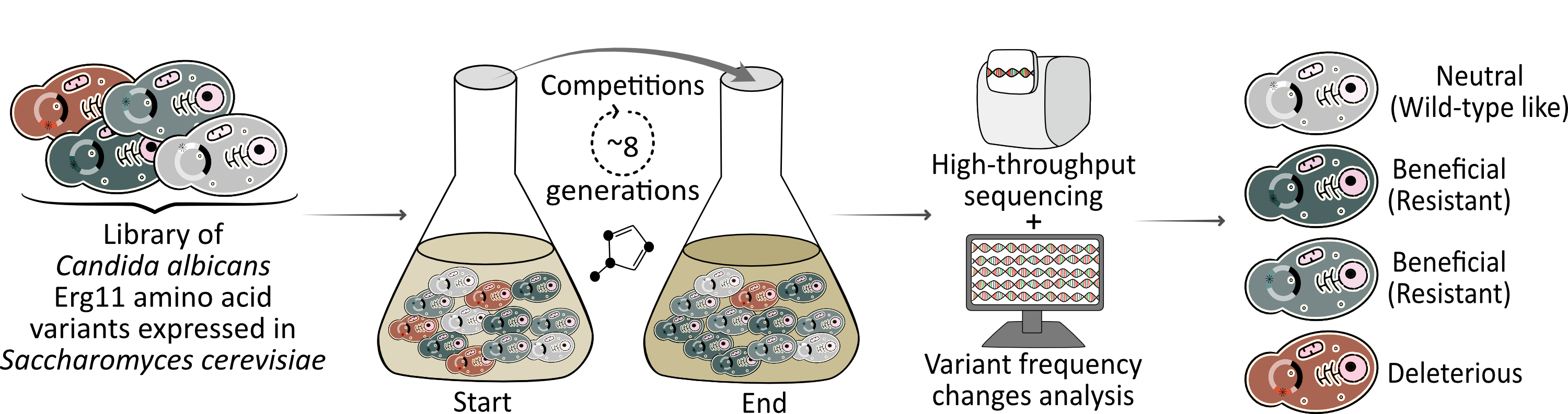
Figure 1: Experimental pipeline to characterize azole resistance mutations in the Candida albicans drug target Erg11. The Erg11 variant library (*) competed for 8 generations in the presence of an azole. High-throughput sequencing at the start and at the end of the competition combined with bioinformatics allowed the phenotypic characterization of all variants.
Many Erg11 mutations confer resistance
From the competitions in the presence of azoles, we found that a large proportion of the mutants conferred resistance, with about a third of the Erg11 variants showing resistance to at least one of the six azoles tested. Next, we wanted to see if we could identify a clear resistance hotspot that would make it easy to interpret mutations in Erg11. To do this, we looked on the 3D protein structure where the resistant mutants were located. Unfortunately, residues with resistance mutations are scattered throughout the structure and are not limited to a single resistance hotspot (Figure 2).
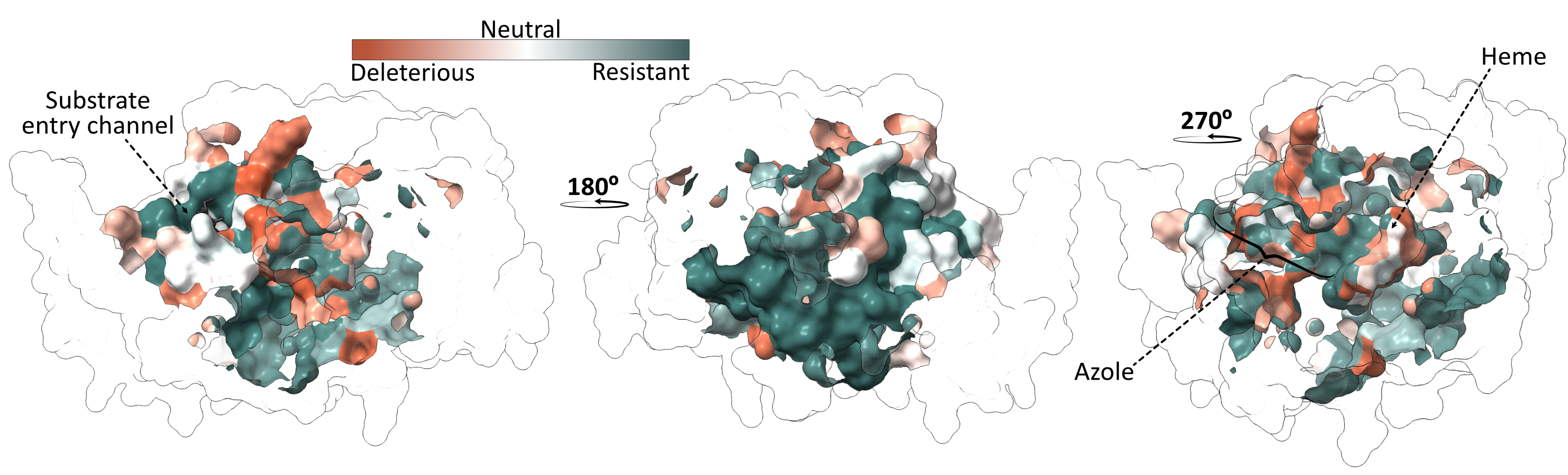
Cross-resistance is the rule, not the exception
After identifying which mutants were resistant to which azoles, we examined whether any mutants conferred resistance to more than one azole simultaneously. We found that many did, as the resistance patterns across the different antifungals are strikingly similar. This was to be expected as all the drugs tested share the same mechanism of action. However, the level of cross-resistance is massive, with almost 90% of resistant variants conferring resistance to more than one azole and more than 60% conferring resistance to all the azoles tested (Figure 3).
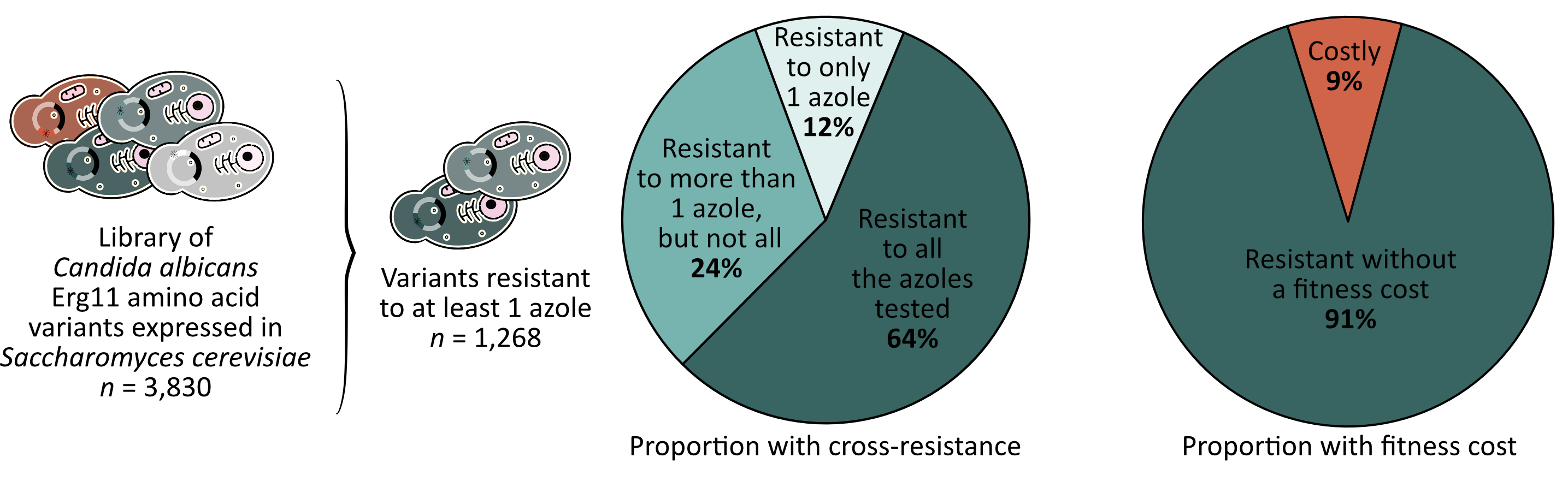
Most of the time, resistance is for free
Finally, we examined whether azole resistant mutants are disadvantaged compared to susceptible mutants in the control condition. We found that very few mutations appeared to affect Erg11 function, resulting in less than 10% of resistant mutants carrying a fitness cost (Figure 3). Since the canonical substrate of Erg11 sits in the same pockets as azoles, we expected that mutations impacting azole binding would also affect the catalytic activity of the enzyme. While our results are consistent with the existing literature, I was surprised by the low proportion of mutants that showed a fitness cost.
Best-case scenario for C. albicans, worst-case scenario for us
When I started this project, I told myself that the worst-case scenario would be a large number of cross-resistance mutations without a fitness cost, and that is exactly what we found. While these results are certainly interesting, they are also quite alarming, as they suggest that C. albicans can adapt to azoles through a wide range of mutations, likely leading to pan-azoles resistance without any associated fitness cost. As fungal infections are a major public health concern, we hope that our new catalog of azole resistance mutations, along with these discoveries, will help direct treatment decisions in the clinic and guide the development of new drugs overcoming resistance.
Warning!!! Cross-resistance between clinical and agricultural azoles?
To my surprise, azole antifungals are not only used in clinical settings but also in agriculture. While the specific azole molecules used in agriculture differ from those used in medicine, they are chemically similar and work in the same way. Unfortunately, there is already evidence that azole-resistant Aspergillus fumigatus from the environment can cause infections in patients, resulting in higher rates of treatment failure3. Given the extent of cross-resistance and the very limited tradeoff we have measured here, the use of azoles in agriculture should be seen as a serious threat to our ability to successfully treat patients using azoles in the clinic.
References
1.World Health Organisation. WHO fungal priority pathogens list to guide research, development and public health action. (2022). at <https://www.who.int/publications-detail-redirect/9789240060241>
2. van Rhijn, N. et al. Beyond bacteria: the growing threat of antifungal resistance. Lancet 404, 1017–1018 (2024).
3. Kang, S. E. et al. Evidence for the agricultural origin of resistance to multiple antimicrobials in Aspergillus fumigatus, a fungal pathogen of humans. G3 Genes|Genomes|Genetics 12, jkab427 (2022).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in