The covert lives of HSCs
Published in Cancer

Explore the Research
Hematopoietic stem and progenitor cell signaling in the niche - Leukemia
Leukemia - Hematopoietic stem and progenitor cell signaling in the niche
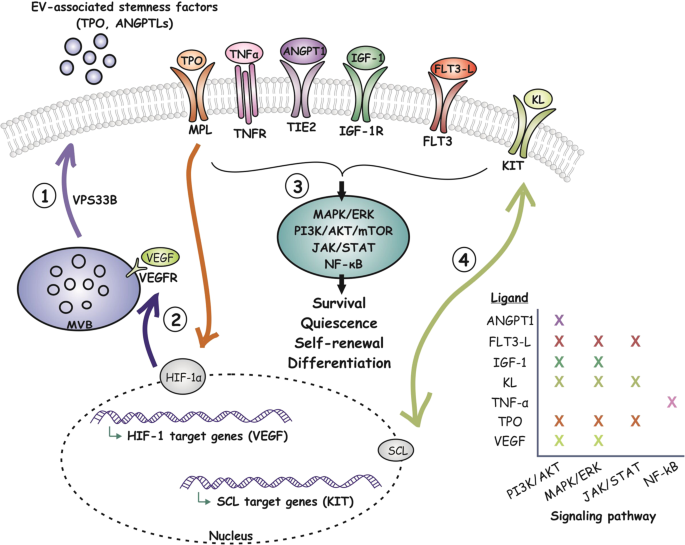
On the heels of recent technological and bioinformatic advances, hematopoiesis research is arguably experiencing a period of unparalleled growth. In vivo imaging and multiplexed cyclic immunofluorescence analysis has helped resolve tissue architecture to subcellular levels. Single cell sequencing and transcription factor occupancy on chromatin provide a glimpse at cellular composition of the bone marrow as well as clonal complexity and regulatory circuitry of the HSC pool. What has been more difficult to capture is the dynamic crosstalk between cells, and in particular the signals that emerge from HSC and their multipotent progeny. Our review of the literature aims to provide a fresh perspective for seemingly disparate observations of HSC signaling via secretion of conventional growth factors and nanosized extracellular vesicles.
The molecular machinery that allows HSC to sense and signal in response to injury or inflammation has long been appreciated. Also uncontroversial is the existence of autocrine loops involving growth factor secretion by HSC. Seminal observations in BM and fetal liver further suggest that HSC signals may indeed actively shape their niche rather than merely passively homing to an inalterable site. And, when viewed as a prototypical HSC-cancer, the acute myeloid leukemia (AML) literature reveals a series of studies and clinical observations to illustrate how cells actively remodel their environment and adapt net hematopoietic output. Understanding the signals that emerge from HSC will not only inform how they compete for occupancy in the leukemic niche, but may also help us formulate strategies to mitigate the bone marrow failure that contributes to morbidity among leukemia patients. Beyond the leukemic niche: what are the signals that transmit relative fitness and drive clonal competition in the HSC pool? How can they be mined to mitigate clonal constraints and selection, for example during aging. Can stem cell transplantation outcomes be improved by directly modulating graft fitness and competitiveness for recipient niches, thereby reducing recipient conditioning intensity or immunomodulation?
Our review argues that HSC actively partake in shaping niche and organ function, and aims to highlight some of the opportunities that come with a more dynamic bidirectional model of HSC signaling.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in