The enormous hydrodynamic forces at the microscale - a key process regulating microbial community structure and ecological characteristics
Published in Microbiology

Imagine a typical day at the office. Everything is going smoothly until, unexpectedly, your coffee takes a tumble, and a droplet splashes onto the paper before you. Annoyed, you witness the liquid slowly disappearing, leaving behind a stain that forms a dark ring-like shape at the droplet's edge. The scientific community refers to this as the coffee ring effect – a process where small, suspended coffee grounds are transported to the droplet periphery and deposited during drying. Interestingly, this phenomenon occurs countless times every second in the world of microbes: when you sneeze, when it rains, when the pores of your skin sweat, or even during the early morning dew. Every time a tiny drop filled with bacterial cells lands on a surface, similar events unfold. But here's the twist: Do these processes matter? Do they influence the way microbial communities work and evolve? And if yes, in what way?
If you delve into the physics underlying the development of a coffee ring, you will uncover a fascinating process. It turns out that the uneven evaporation across the droplet surface is the driving force behind this phenomenon. This creates microscale flows within the droplet, originating from the droplets' center and extending towards the edges. Similar to the coffee grounds, this flow transports bacterial cells toward the droplet edges, where they accumulate and ultimately give rise to the characteristic ring-like structure. However, a completely different scenario unfolds when the droplet contains surfactants that are commonly observed in nature and even produced by bacteria. In this case, the droplets evaporate more evenly and give rise to the formation of a more circular flow termed Marangoni convection. Consequently, when a droplet lands on a surface, diverse hydrodynamic regimes driven by evaporation come into play, shaping the initial distribution of bacteria within the colony. But what happens once the bacterial cells are deposited on a surface? Individual cells begin to grow and divide, during which they rapidly fill the space around them and collectively start to expand radially as a colony (a process called range expansion). During this process, only a thin band of cells at the colony periphery have access to abundant nutrients and space to expand, creating a strong selective force restricting which cell lineages can persist, and which cell lineages are ultimately outgrown by their neighbors and “stuck” behind the expanding front.
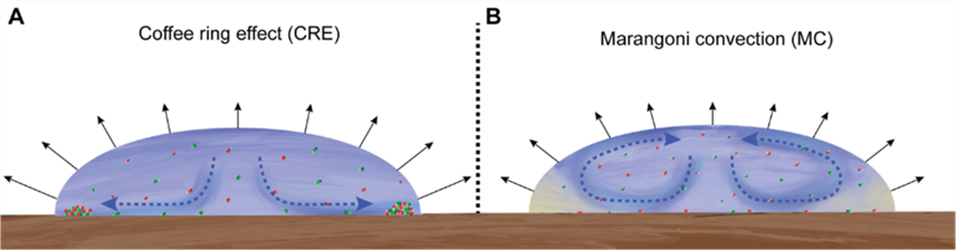
Fig. 1: Conceptual description of cell deposition under different evaporation-induced hydrodynamics. A) The coffee ring effect (CRE) is caused by preferential evaporation along the surface-pinned liquid-air interface (the contact line). This causes capillary flows toward the contact line and the accumulation of cells at the droplet periphery. B) Marangoni convection (MC) is caused by surfactant accumulation at the contact line that reduces the differential effect of evaporation across the liquid-air interface (illustrated here as the yellow gradient). This causes the formation of circular vortices that redistribute cells and result in a more homogeneous cell deposition pattern.
Based on this knowledge, one would assume that different deposition mechanisms (coffee ring pattern versus Marangoni convection) play a crucial role in how the bacterial community develops and how bacterial strains within the community interact!
Setting out to explore this interplay between the initial deposition pattern and community development, we examined how different evaporation-induced hydrodynamic processes including the coffee ring effect (CRE) or Marangoni convection (MC) influence the diversity within the emerging colonies and contact-dependent processes such as plasmid transfer between strains. By enhancing Marangoni convection (MC) via the addition of a biologically inert surfactant, we controlled the balance between evaporation-induced hydrodynamic flows in the inoculation droplet. We then observed the growth of a single-species bacterial colony and the persistence of individual lineages that express different fluorescent proteins within the colony. When MC dominates the flows, bacterial cells in the inoculum are evenly spread across the droplet area which means that the cell density in the expanding front is initially lower, and fewer cell lineages proliferate. In the case of the CRE, cells within the droplet are concentrated at the periphery during droplet drying, resulting in a high density of cells within the expanding front and numerous lineages persisting during range expansion.
In addition to the overall maintenance of diversity at the level of individual lineages, the different expansion patterns induced by the CRE and MC also conceivably alter the dynamics of contact-dependent cell processes such as horizontal gene transfer. We combine two species that express different fluorescent proteins where one strain (called the donor strain) additionally carries a plasmid that encodes for an additional fluorescent protein. The second strain (called recipient strain) can obtain the plasmid from the donor strain via conjugation and subsequently express both fluorescent proteins such that we can distinguish the three cell types (donor, recipient, and transconjugant) via fluorescence microscopy. In the case of the CRE that concentrates cells at the edge, the higher intermixing of individual lineages yields a greater extent of plasmid transfer and overall transconjugant cell population (cells that obtained a plasmid during range expansion, Fig. 2). Conversely, the more even cell distribution driven by the MC results in less plasmid transfer since there is less contact between potential plasmid donor and recipient strains (Fig. 2). Therefore, evaporation-driven hydrodynamic flows significantly impact the spatial organization of range-expanding bacterial colonies and, consequently, the potential for plasmid transfer, processes that are crucial to driving adaptation, evolution, and functional innovation.
Beyond scientific discovery lies the promise of transformative applications. By harnessing the knowledge of how microscale hydrodynamics influence the development and functioning of microbial communities, we can engineer microbial systems and tailor them for optimal functionality to craft novel solutions for challenges ranging from environmental remediation to biotechnology. In short, insights from this research reverberate far beyond the confines of the laboratory and pave the way we interact with and harness the microbial world.
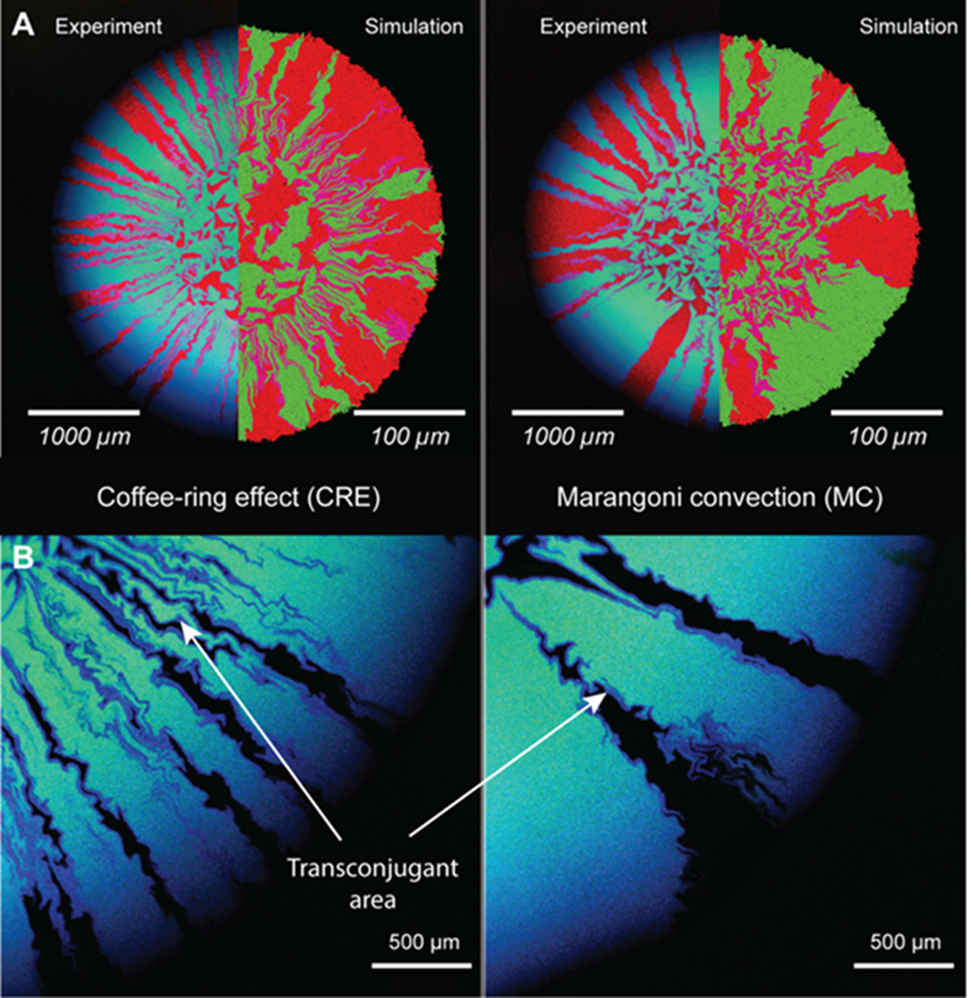
Fig. 2: Range expansion and plasmid transfer during surface-associated growth after cell deposition under different evaporation-induced hydrodynamics. A) Images are for cell deposition dominated by the coffee-ring effect (left) or Marangoni convection (right). For the upper panels, the experiments are shown in the left hemispheres, and simulations from an individual-based model are shown in the right hemispheres. Here, the recipient strain expresses a red fluorescent protein whereas the donor strain expresses a green fluorescent protein in addition to a blue fluorescent protein encoded by the plasmid. If the recipient strain successfully receives the plasmid (then called transconjugant cell), it expresses both red and blue fluorescent proteins and thus appears in magenta. B) Images of successful plasmid transfer in the experiments. In these images, the red channel was removed to improve visualization of the transconjugants, which appear as blue. Note that transconjugants emerge along the interfaces between the donor and recipient strains.
Authors: Chujin Ruan, Benedict Borer, Josep Ramoneda, Gang Wang, David R. Johnson
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: May 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in