The increasing prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD) has become a global public health problem. Alterations in the gut microbiota are believed to contribute to MAFLD. There is increasing evidence supporting the use of prebiotics like oligosaccharide to alleviate MAFLD by modulating gut microbiota, though the detailed mechanisms are not fully known. Inulin, a commonly used prebiotic, is reported to have various beneficial functions on the host, such as regulation of lipid metabolism, lowering blood sugar and losing weight. As an indigestible dietary fiber, the health-improving effects of inulin are mainly attributed to its role in modulating gut microbiota, especially in promoting the growth of traditionally recognized probiotics such as Lactobacillus and Bifidobacterium spp. However, if and how inulin interacts with other gut commensal microbes and thus benefit the host metabolisms still needs to be elucidated.
Currently, diet-induced MAFLD rodents and genetically modified mice are the most widely used animal models. However, liver and adipose tissue in rodent models contribute equally to de novo lipogenesis, and rodents have a lower incidence of MAFLD than humans during the natural processes of life. Comparatively, the chicken liver bears more than 90% de novo lipogenesis, which is very similar to that of humans. In addition, excessive hepatic fat deposition and susceptibility to MAFLD are common pathological phenomena in laying hens during normal aging. Therefore, laying hens attract our interest as an animal model for understanding and studying the role of inulin in human MAFLD.
We found that inulin effectively ameliorated metabolic disorders in the HFD-fed hens, and Megamonas was subjected to the most dramatic changes before and after inulin intervention in the hens’ ceca. Correlation analysis found that Megamonas was significantly negatively correlated with hepatic steatosis-related parameters, and the negative correlation between Megamonas species and chicken liver lipid content was further validated by an independent cohort of old laying hens and FMT experiment (data not shown). These results prompted us to investigate the direct effect of Megamonas on MAFLD. We then isolated Megamonas species directly from the cecal contents of hens with inulin intervention using culturomics method. Obtaining the target bacterium from the numerous microbes in gut is a time-consuming and painstaking job. Xinyue Yang, the first author of this paper, picked ~3,000 individual bacterial colonies from the agar plates and identified 193 bacterial species belonging to 5 phyla. The target species, Megamonas funiformis (strain CML154) was finally purified and the whole genome of this strain was sequenced. We further demonstrated that inulin could stimulate the growth of M. funiformis CML154 in a dose-dependent manner.
We next gavaged M. funiformis CML154 to the MAFLD laying hen model, and verified the role of this strain in attenuating MAFLD. Interestingly, although strain CML154 is isolated from laying hens, it also works in attenuating MAFLD in mice model, displaying a cross-species effect in the regulation of host lipid metabolism. How does M. funiformis CML154 regulate lipid metabolism? We found that strain CML154 is a short chain fatty acids (SCFAs) producer, with the major product of propionic acid. All our in vivo results confirmed that both inulin and strain CML154 intervention increased the propionate concentration in the host gut. Mechanisms involved in the propionate-mediated lipid-lowering effect was further explored using hepatocyte model of hepatic steatosis in a Transwell co-culture system. Combing all the in vivo and in vitro results, we demonstrated that the anti-MAFLD effect of M. funiformis CML154 is due to the activation of the APN-AMPK-PPARα signaling pathway by its production of propionic acid (Figure 1).
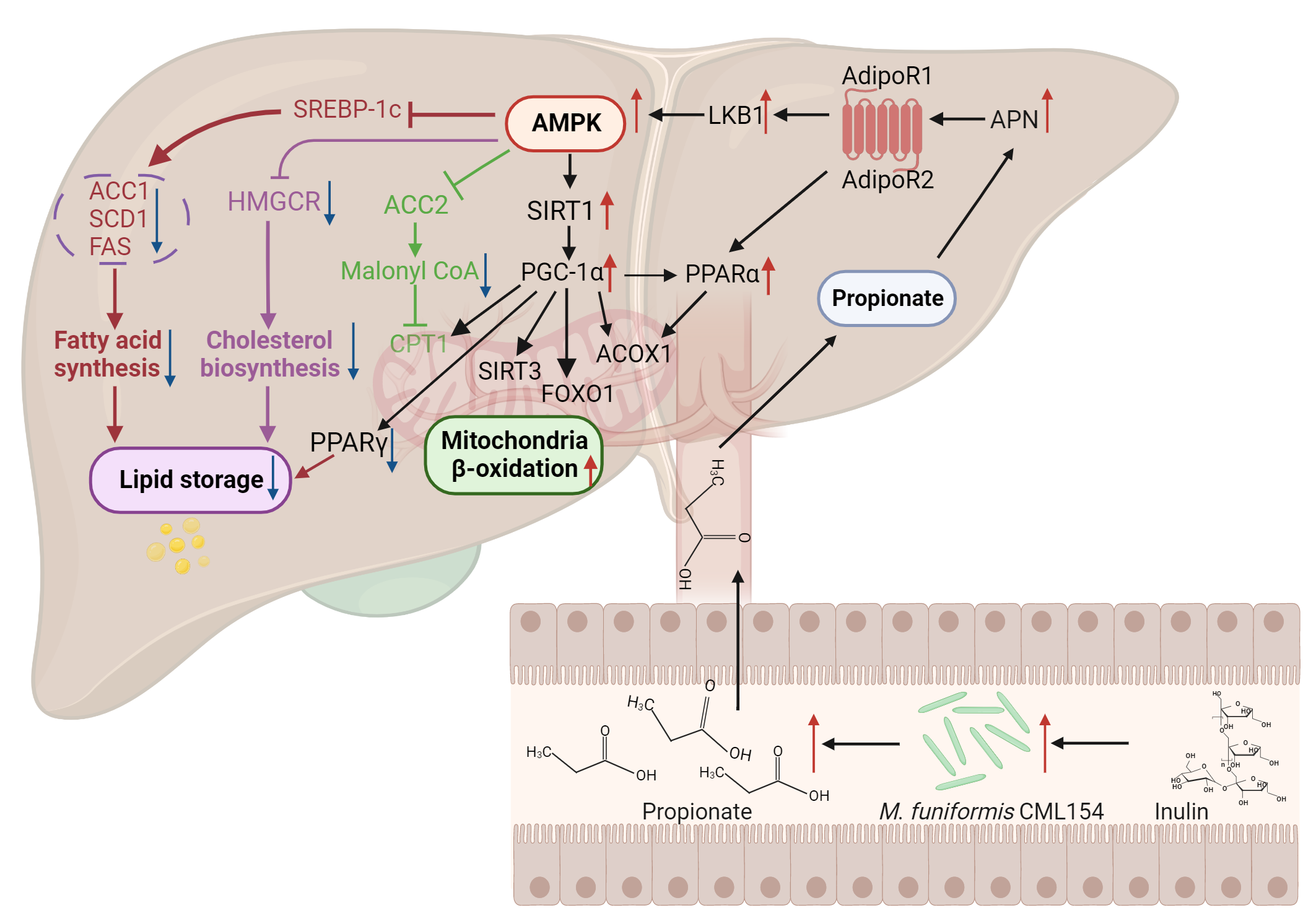
Figure 1 Proposed mechanisms underlying the anti-MAFLD effect of inulin mediated by M. funiformis CML154.
Overall, we provide compelling evidence linking the role of inulin in alleviating MAFLD with the performance of M. funiformis CML154, a potential probiotic bacterium for the prevention and treatment of MAFLD and related metabolic diseases. More than that, our study proved that chicken, especially the laying hen, is an appealing animal that can be used for understanding and investigating MAFLD in humans.
For more details on this work, visit our recent publication in npj Biofilms and Microbiomes: https://www.nature.com/articles/s41522-023-00451-y.
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: Feb 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in