The Sugar Crush of the Fungal Toxin Candidalysin
Published in Microbiology, Cell & Molecular Biology, and Biomedical Research
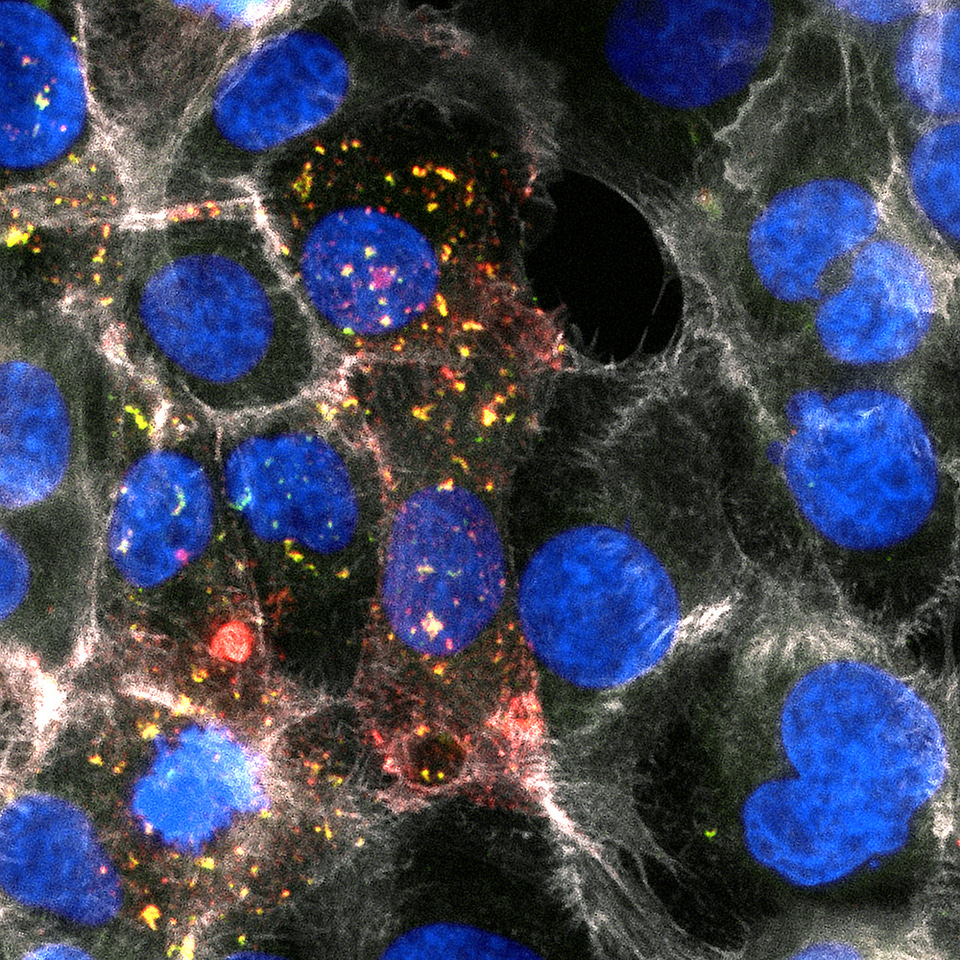
Fungal infections often fly under the radar, yet they pose a significant threat to global health. Recent research estimates that more than 3.5 million people die from fungal infections each year worldwide 1. Despite this high mortality, the severity of fungal diseases is often overlooked. In a landmark move, the World Health Organization (WHO) recently published its first fungal priority pathogen list, highlighting 19 fungal pathogens that require urgent research and attention. Among the most critical of these pathogens is Candida albicans, a fungus responsible for serious infections like invasive candidemia and chronic mucosal candidiasis.
The economic impact is substantial. In the USA alone, annual healthcare cost for Candida infections is around $2 billion 2, with similar per capita costs in the European Union. C. albicans accounts for ~75% of all Candida infections and is an enormous global health burden, the severity of which continues to escalate.
Our research group, led by Dr. Scott Filler at the Lundquist Institute and David Geffen School of Medicine at UCLA, focuses on fungal-host interactions. Candidalysin, a cytolytic peptide toxin first identified by the Naglik and Hube labs in 2016 3, is a critical virulence factor in mouse models of disseminated, vaginal, and oropharyngeal candidiasis. Although much has been learned about candidalysin in subsequent years, one mystery remained: what is the host cell target of candidalysin? Our latest study, ‘Sulfated glycosaminoglycans are host epithelial cell targets of the Candida albicans secreted toxin candidalysin’ published in Nature Microbiology, reveals sulfated glycosaminoglycans to be the host cell targets of candidalysin.
Paradigm shift: the host ligands of candidalysin are sugars, not proteins
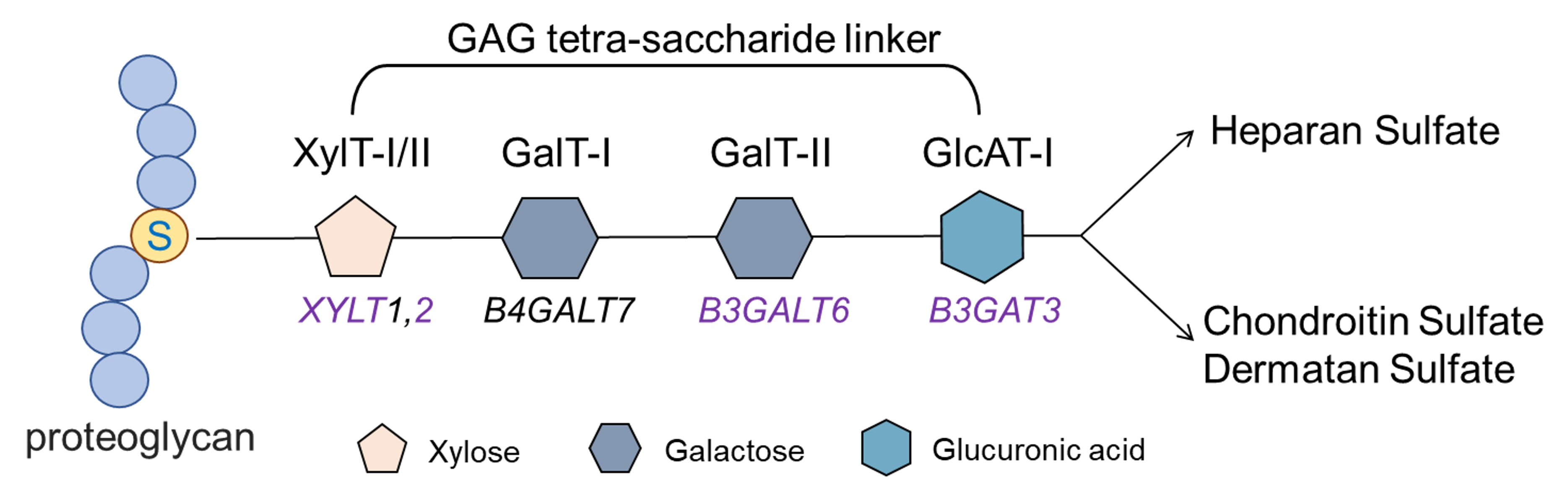
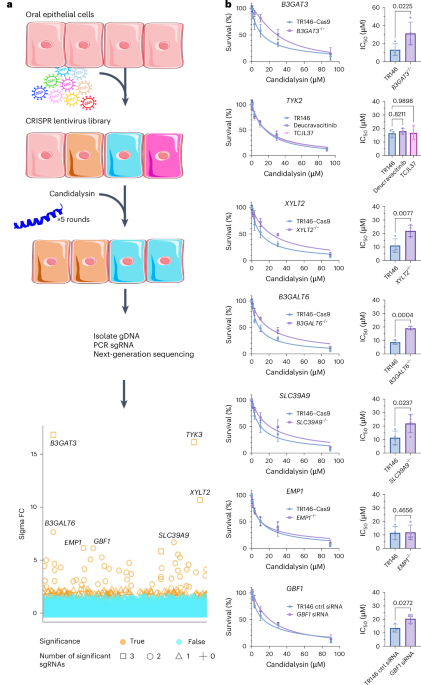
My journey to uncover the host ligand for candidalysin was anything but straightforward. I embarked on this project in March 2020, right as the COVID-19 pandemic began to disrupt research worldwide. Like many other labs, we faced challenges due to social distancing measures and supply shortages. Our initial hypothesis was that candidalysin would interact with protein receptors on the cell membrane, and we used a CRISPR screen to identify potential candidates. I focused on genes coding for membrane proteins, expecting that disrupting these genes would make cells resistant to candidalysin. However, after testing several candidates without success, I revisited the list and noticed a pathway involved in the synthesis of glycosaminoglycans (GAGs). GAGs are linear polysaccharides such as heparan sulfate, chondroitin sulfate, and dermatan sulfate that are linked to proteins on the host cell surface. The enzymes I discovered in my CRISPR screen synthesize the tetra-saccharide (a chain of four sugars) that links GAGs to the core proteins (Figure 1).
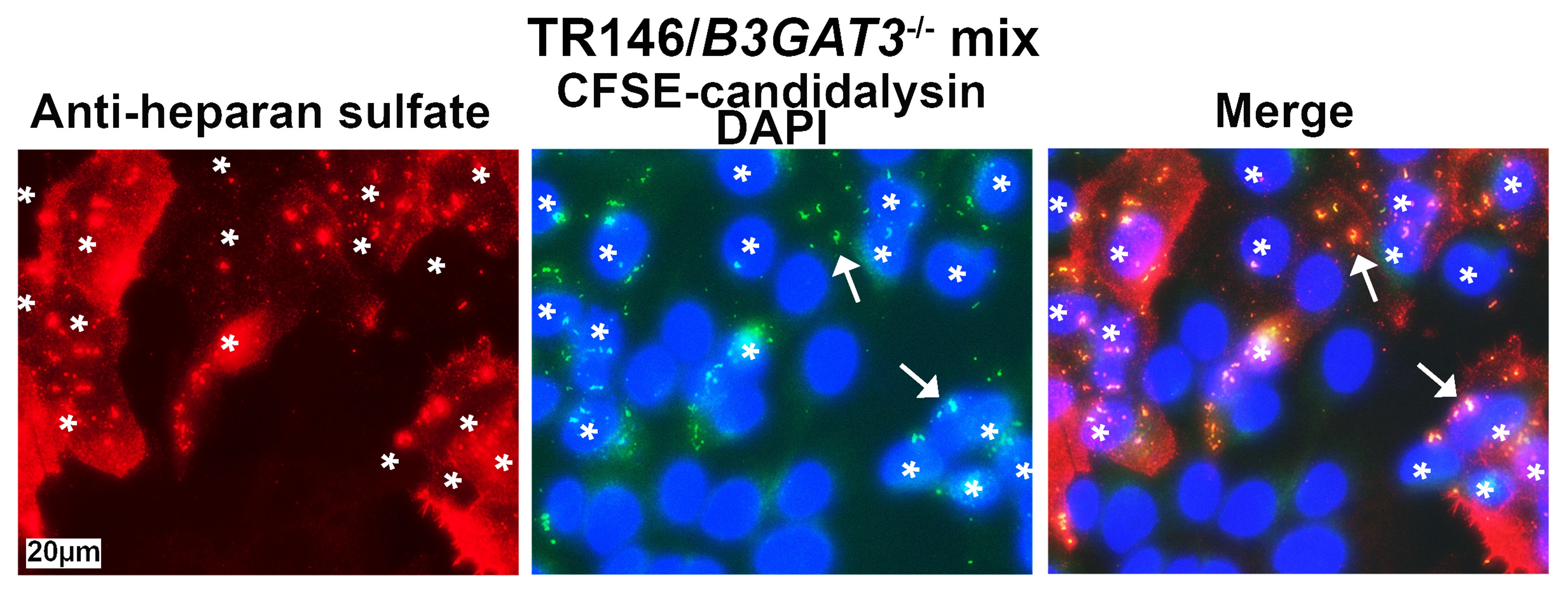
Through immunofluorescence assays, I observed that candidalysin interacted with GAGs on the cell surface. This was a breakthrough: the host ligand for candidalysin wasn't a protein, but sugars that were attached to proteins. Candidalysin bound to cells that had these GAGs (Figure 2).
Collaborations and Discoveries: Uncovering the Candidalysin-GAG Interaction
Our understanding of the candidalysin-GAG interaction deepened through collaboration. During the pandemic, many researchers, including our collaborator Dr. Fuming Zhang at Rensselaer Polytechnic Institute, focused on studying the SARS-CoV-2 virus, leading to many high-quality publications. Dr. Zhang, an expert on polysaccharides such as GAGs, helped measure the dynamics of the interactions of candidalysin with the GAG, heparin. We found that this interaction is remarkably stable, with a half-life of over 50 minutes.
In 2022, Dr. Francisco Barrera and Dr. Gavin King published a paper in eLife that revealed the structures of pores formed by candidalysin on artificial membranes 4. I reached out to these authors, and they were happy to collaborate with us study the biophysics of the candidalysin-GAG interaction. Their experiments showed that GAGs facilitate the clustering (oligomerization) of candidalysin, which is crucial for its function.
Dextran Sulfate: A Potential "Silver Bullet"
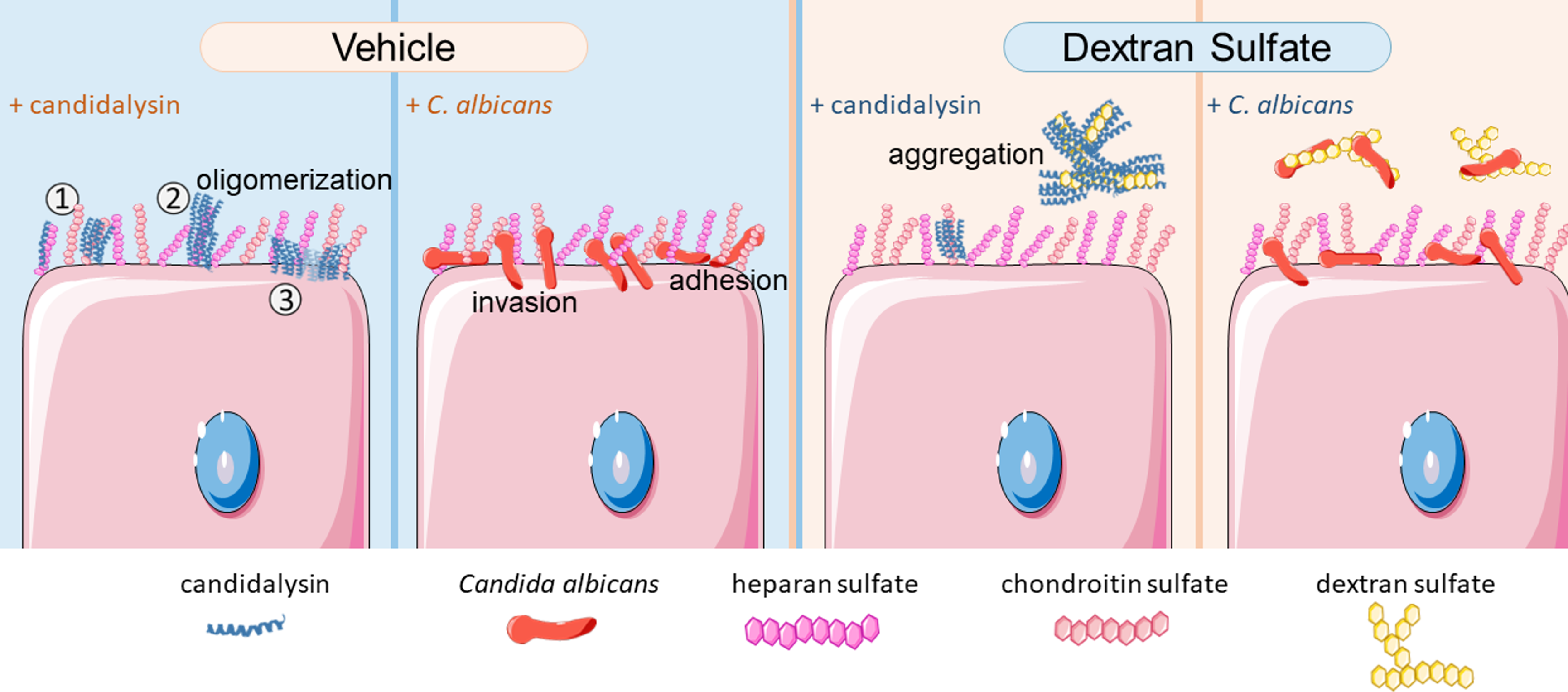
In competition assays, I discovered that adding external GAGs could protect host cells from damage caused by candidalysin. Interestingly, GAG analogues like dextran sulfate and α-cyclodextrin also provided similar protection, as long as these polysaccharides were sulfated. Dextran sulfate, a plant-derived polysaccharide not naturally found in humans or animals, emerged as a promising therapeutic agent. Being inert to the human immune system makes dextran sulfate advantageous as a potential treatment. Exogenous dextran sulfate not only shielded host cells from candidalysin-induced damage but also protected them against live Candida albicans cells. Its protective effects were multifaceted: it reduced fungal adherence and invasion of host cells, dampened immune responses, and minimized cell damage (Figure 3).
In the mouse model of vulvovaginal candidiasis, topical application of dextran sulfate reduced tissue damage and inflammation. This discovery underscores the potential of sulfated GAGs as therapeutic agents to protect against candidalysin-induced damage.
Conclusion
Our research highlights a shift in understanding how fungal toxins like candidalysin interact with host cells. Instead of proteins, sugars play a critical role in this interaction, opening new avenues for therapeutic interventions. As fungal infections continue to pose a global health challenge, innovative approaches like the use of dextran sulfate could offer new hope for prevention and treatment.
References
1 Denning, D. W. Global incidence and mortality of severe fungal disease. Lancet Infect Dis, doi:10.1016/S1473-3099(23)00692-8 (2024).
2 Benedict, K., Jackson, B. R., Chiller, T. & Beer, K. D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin Infect Dis 68, 1791-1797, doi:10.1093/cid/ciy776 (2019).
3 Moyes, D. L. et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532, 64-68, doi:10.1038/nature17625 (2016).
4 Russell, C. M. et al. The Candida albicans virulence factor candidalysin polymerizes in solution to form membrane pores and damage epithelial cells. Elife 11, doi:10.7554/eLife.75490 (2022).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in