Topical application of calcitonin gene-related peptide as a regenerative, antifibrotic, and immunomodulatory therapy for corneal injury
Published in Neuroscience, Biomedical Research, and General & Internal Medicine

Corneal opacity is a leading cause of visual impairment, affecting more than 5.5 million people worldwide [1]. Although corneal opacity is associated with several underlying etiologies, trauma accounts for nearly one-third of all cases [2]. Current therapeutic modalities for moderate to severe corneal opacity are limited to corneal transplantation with issues such as limited tissue supply, surgical complications, and graft failure . Therefore, there is an urgent need to develop alternative therapies that can efficaciously prevent corneal opacification and promote wound healing and tissue regeneration.
The cornea is the most densely innervated tissue in the human body with sensory nerves derived from the ophthalmic branch of the trigeminal nerve (cranial nerve V1). The role of corneal nerves in wound healing has been reported [3 4]. Additionally, it was reported that sensory nerve degeneration and dysfunction in diabetic and neurotrophic keratopathy can lead to recurrent ulceration, delayed wound healing and corneal opacification [5]. Corneal nerves secrete neuropeptides that carry out regulatory functions at the ocular surface [6]. One of these neuropeptides is calcitonin gene-related peptide (CGRP), which is expressed by two-thirds of corneal nerves [7].
CGRP has been demonstrated to accelerate wound healing and inhibit tissue fibrosis in the skin and bronchial epithelium [8-10]. Furthermore, its absence (in CGRP knockout mice or antibody-mediated blockade) results in delayed wound closure [11] and accentuated inflammation [12]. Yet, the efficacy of topically applied CGRP in promoting corneal wound healing and regeneration and treating corneal stromal injury remains unknown.
In this research report, we evaluated the effect of CGRP on corneal wound healing following mechanical injury. We found that corneal injury leads to nerve damage and depletion of neuropeptides including CGRP. Topical application of CGRP as an eye drop accelerates corneal epithelial closure, preserves corneal transparency, and prevents scar formation and edema. Mechanistically, CGRP promotes corneal epithelial cell migration, proliferation, and the secretion of the basement membrane; it reduces TGF-β1-mediated stromal fibroblast activation and tissue fibrosis; CGRP preserves corneal endothelial density and function; and lastly, it reduces neutrophil infiltration, macrophage maturation, and the production of inflammatory cytokines. Taken together, our results show that corneal nerve-derived CGRP plays a regenerative, anti-fibrotic, and anti-inflammatory role in corneal wound healing and that corneal innervation is a key regulator of ocular surface homeostasis and injury repair.
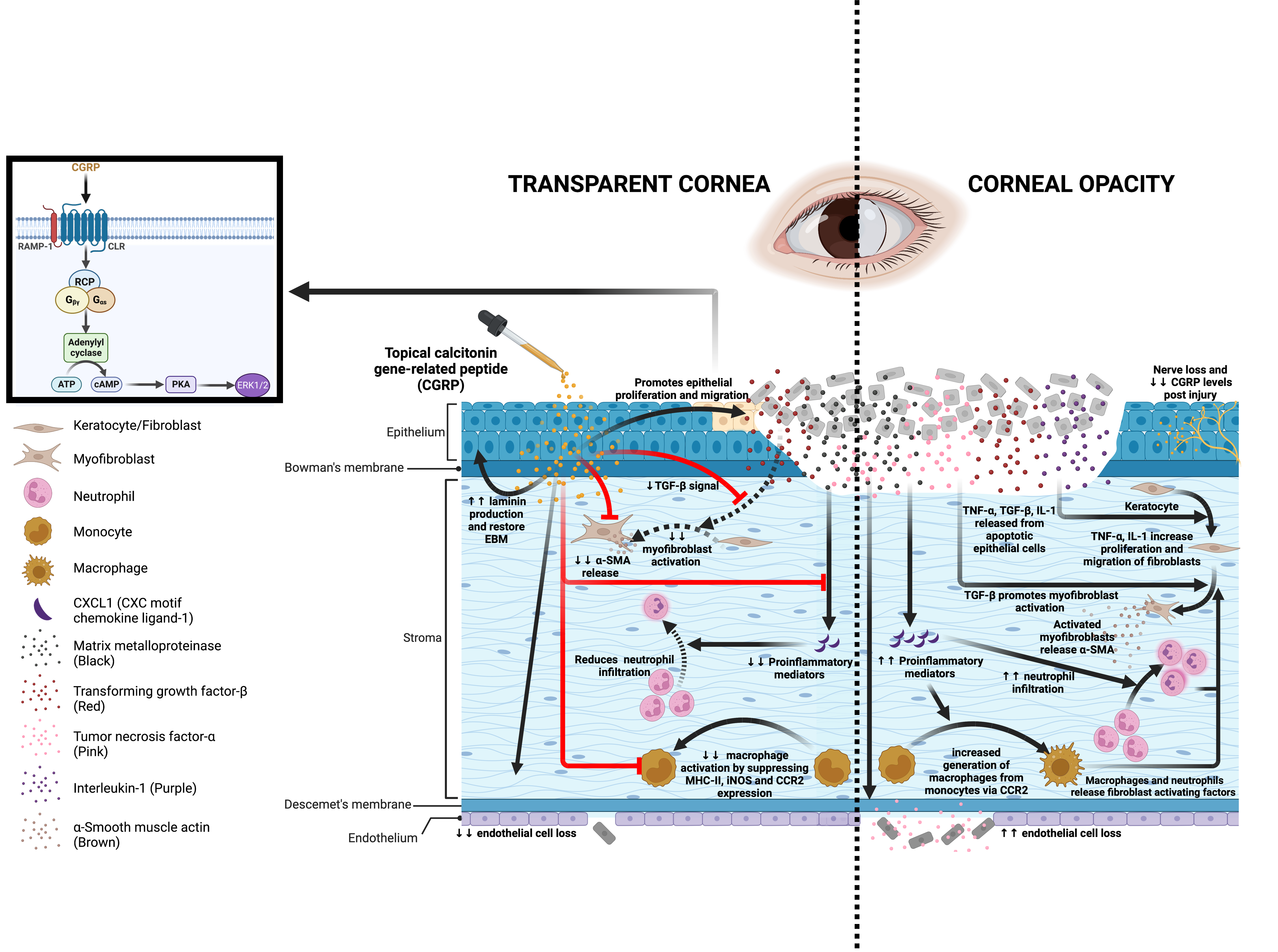
Schematic showing the effects of CGRP on corneal wound healing after mechanical injury.
Injury leads to nerve damage and a decrease in CGRP level in the cornea. Topical application of CGRP promotes corneal epithelial cell regeneration and restores the epithelial basement membrane, thus reducing the release of pro-inflammatory and pro-fibrotic mediators including TNF-α, TGF-β, IL-1, and CXCL1 into the stroma. This leads to reduced keratocyte activation and stromal fibrosis. In addition, CGRP reduces neutrophil infiltration, macrophage maturation, and the production of inflammatory cytokines. It reduces corneal endothelial cell loss and maintains its pump function. Clinically, topical application of CGRP as an eye drop accelerates epithelial closure, preserves transparency, and prevents scar formation and edema after corneal injury.
References:
- Wang E, Kong X, Wolle M, et al. Global trends in blindness and vision impairment due to corneal opacity 1984-2020: a meta-analysis. Ophthalmology 2023 doi: 10.1016/j.ophtha.2023.03.012[published Online First: Epub Date]|.
- Jeng BH, Ahmad S. In Pursuit of the Elimination of Corneal Blindness: Is Establishing Eye Banks and Training Surgeons Enough? Ophthalmology 2021;128(6):813-15 doi: 10.1016/j.ophtha.2020.06.042[published Online First: Epub Date]|.
- Wu M, Hill LJ, Downie LE, Chinnery HR. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Progress in Retinal and Eye Research 2022;91:101105 doi: https://doi.org/10.1016/j.preteyeres.2022.101105[published Online First: Epub Date]|.
- Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic Influences on Corneal Epithelial Cells. Experimental Eye Research 1994;59(5):597-605 doi: https://doi.org/10.1006/exer.1994.1145[published Online First: Epub Date]|.
- Yu F-sX, Lee PSY, Yang L, et al. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Progress in Retinal and Eye Research 2022;89:101039 doi: https://doi.org/10.1016/j.preteyeres.2021.101039[published Online First: Epub Date]|.
- Puri S, Kenyon BM, Hamrah P. Immunomodulatory Role of Neuropeptides in the Cornea. Biomedicines 2022;10(8) doi: 10.3390/biomedicines10081985[published Online First: Epub Date]|.
- Zhu S, Zidan A, Pang K, Musayeva A, Kang Q, Yin J. Promotion of corneal angiogenesis by sensory neuron-derived calcitonin gene-related peptide. Experimental Eye Research 2022;220:109125 doi: https://doi.org/10.1016/j.exer.2022.109125[published Online First: Epub Date]|.
- Zhou Y, Zhang M, Sun G-Y, et al. Calcitonin gene-related peptide promotes the wound healing of human bronchial epithelial cells via PKC and MAPK pathways. Regulatory Peptides 2013;184:22-29 doi: https://doi.org/10.1016/j.regpep.2013.03.020[published Online First: Epub Date]|.
- Toda M, Suzuki T, Hosono K, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomedicine & pharmacotherapy 2008;62(6):352-59
- Zhang Y, Gao N, Wu L, et al. Role of VIP and sonic hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic corneas. Diabetes 2020;69(7):1549-61
- Wurthmann S, Nägel S, Hadaschik E, et al. Impaired wound healing in a migraine patient as a possible side effect of calcitonin gene-related peptide receptor antibody treatment: a case report. Cephalalgia 2020;40(11):1255-60
- Ray JC, Allen P, Bacsi A, et al. Inflammatory complications of CGRP monoclonal antibodies: a case series. The journal of headache and pain 2021;22(1):1-8
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in