Towards saving the failing heart with stem cell therapy
Published in Bioengineering & Biotechnology

The paper in Nature Biomedical Engineering is here: http://go.nature.com/2HdSlch
Almost ten years ago, when I moved my laboratory from India to Stanford, I was searching for a clinically oriented collaborator who could translate formulation and drug delivery ideas to therapy for patients with a failing heart in chronic health conditions. This was an emotional decision for me: around that time, my mother had passed away due to a heart attack. There was nothing we could do about her failing heart. How big of a difference would it have made if we had the ability to help patients like my mother by repairing heart tissue and improving their quality of life?
Fortuitously, Dr. Joseph C. Wu, then a young faculty member at the Stanford School of Medicine, was looking for a formulation scientist like me to improve the survival of intracoronary injected stem cells for treatment of heart disease. This was an important objective, as only 1-5% of injected cells have been shown to persist in animal models receiving cardiac cell grafts1. The Wu lab’s approach was to mix the cells with various growth factors and then track their survival by bioluminescence imaging with the firefly luciferase reporter gene. Eventually we came up with a recipe with three growth factors: EPO, FGF2 and BMP2.
As a formulation and drug delivery scientist, I joined the program with limitless enthusiasm. At first, we found out that these three growth factors were sufficient to improve adult stem cell survival in a murine subcutaneous model, but that the effects were temporary due to the short half-life of the growth factors. As such, we faced an uphill task of manufacturing an FDA-compliant biomaterial that could prolong the growth factors release in vivo. With my background in collagen, an amazing nontoxic, FDA-approved molecule that can bind a plethora of indigenously expressed molecules to form a “live corona”2, we decided to use a collagen-based material for delivery. Our objective was for the biomaterial to work as both a ‘home’ and a ‘collector trap’ for retaining growth factors.
We faced challenges with manufacturing the growth factors, which we found were too large to be protected against degrading enzymes naturally found in the body. One idea was to encase the growth factors within the collagen microfibrils. In our search for ‘tiny’ growth factors, we found smaller peptide analogues, one of which was FDA approved. We linked these peptides with dendrimers using EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide) chemistry with collagen and injected them along with the stem cells. This led to a breakthrough in improving survival of the injected stem cells (Fig. 1).
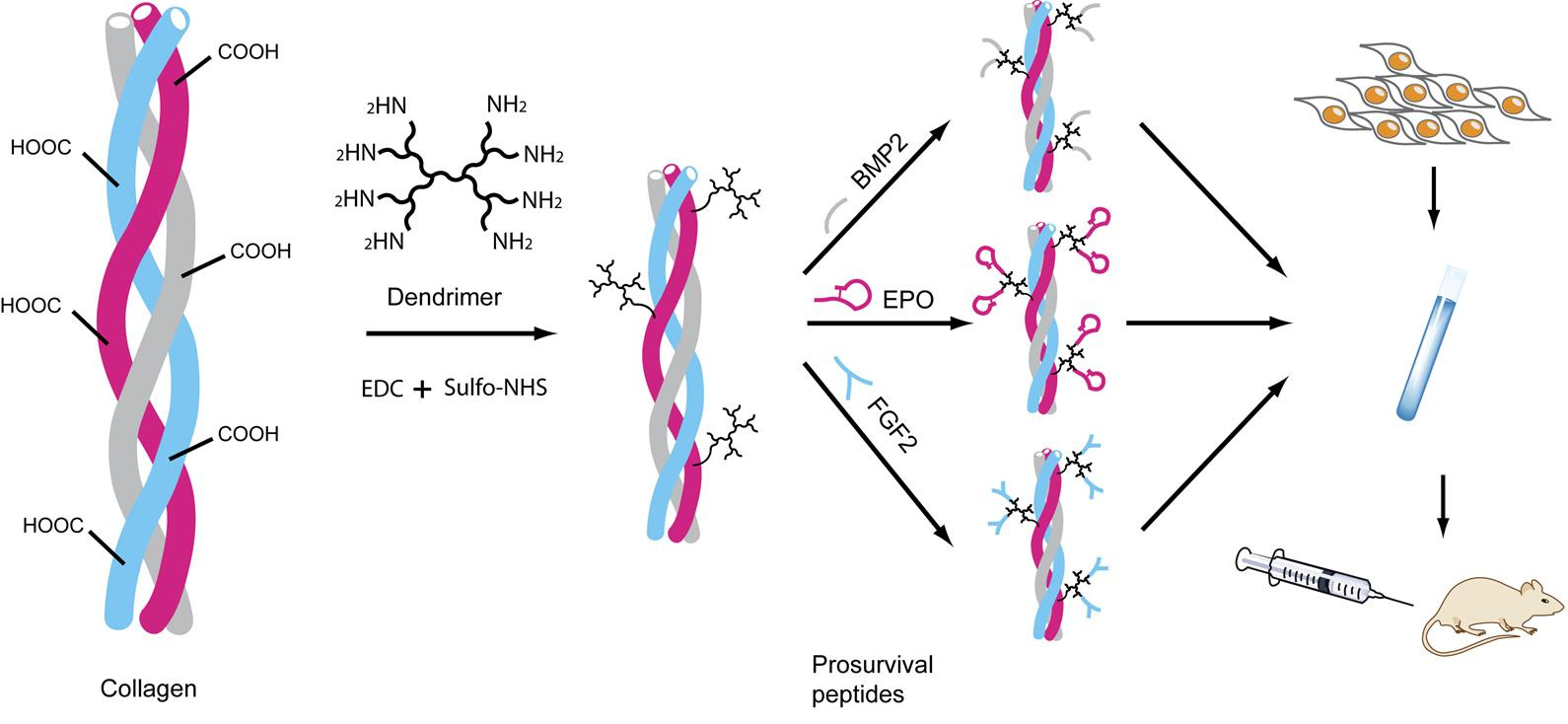
Fig. 1. A schematic depicting the process of col×D×pep synthesis, crosslinking peptide analogs of growth factors via dendrimers. Dendrimer is first crosslinked to collagen using EDC/Sulfo-NHS to prepare col×D. Three pro-survival peptides are then crosslinked to col×D separately and mixed together to prepare the pro-survival collagen matrix. Adapted from ref. 3. Macmillan Publishers.
Currently, we are developing formulation changes to tailor the survival factors for humans and make them work as well as they did in the mouse model. We believe that with this paper we have demonstrated new ways of improving injected stem cell survival in the infarcted heart.
Our paper: Lee, A. S. et al. Prolonged survival of transplanted stem cells after ischemic injury via the slow release of pro-survival peptide analogs crosslinked to an injectable collagen matrix. Nat. Biomed. Eng. 2, 104-113, 2018.
References
1. Sanganalmath, S. K. & Bolli, R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 113, 810-834, 2013.
2. Serpooshan, V. et al. Protein corona influences cell-biomaterial interactions in nanostructured tissue engineering scaffolds. Adv. Funct. Mater. 25, 4379-4389, 2015.
3. Lee, A. S. et al. Prolonged survival of transplanted stem cells after ischemic injury via the slow release of pro-survival peptide analogs crosslinked to an injectable collagen matrix. Nat. Biomed. Eng. 2, 104-113, 2018.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in