Transatlantic nanosensor engineering
Published in Electrical & Electronic Engineering

In summer 2017, Sangeeta Bhatia from MIT visited Imperial College London and presented on her group’s diagnostic technology: nanoparticle sensors that are injected into the blood stream, home to the disease environment (e.g. tumor site), and are disassembled by disease-specific proteolytic enzymes to release reporter probes that are small enough to filter through the kidneys and accumulate in the urine. Reporter probe concentration can then be measured in the urine and correlated to enzyme activity as an indicator of disease state. The Bhatia group had previously applied this platform for detection and monitoring of a variety of conditions, including cancer1,2 and bacterial infections.3 However, to date, the diagnostic readouts required some form of sample processing or expensive equipment that would be difficult to use outside of a laboratory or hospital setting.
At the same time, Molly Stevens’ group at Imperial had been thinking about ways to bring sensitive diagnostic tests to resource-limited settings. In the Stevens group, we were working on developing a class of nanomaterials that could be used to amplify signal and improve the sensitivity of simple paper-based diagnostic tests. We developed a diagnostic platform, similar to a home pregnancy test, that utilizes catalytic nanoparticles for simple and sensitive detection of protein biomarkers via the generation of a color change for simple visual detection.4,5
Inspired by Sangeeta’s lecture, we (Colleen; who had been working on catalytic nanomaterials for biosensing at Imperial, and Ava; who had been developing tools to probe protease activity in disease at MIT) – connected and began to envision how we might be able to incorporate the catalytic nanomaterials at Imperial into the in vivo sensing technology developed at MIT. In this system, sensors would be introduced into the bloodstream, and catalytic nanoparticles would be released into the urine only in the presence of disease-associated enzymes, followed by a quick colorimetric assay on the urine (< 30 min). The result would be a simple color-change urine test, where the development of a blue colored dye in the urine would indicate the presence of a disease.
Before designing the sensing system, we ran a few pilot experiments to see if 1) we could synthesize protease-responsive catalytic nanoparticles that were small enough (<5 nm) to clear into the urine and 2) whether the particles retained their catalytic activity in the urine after exposure to complex physiological environments in vivo. What we found was that we could synthesize peptide-functionalized gold nanoclusters (AuNCs) that were responsive to protease activity, and that, through pharmacokinetic experiments in mice, these nanoclusters cleared into the urine where they maintained their catalytic activity.
After these critical “go or no-go” pilot experiments, we next moved on to the development of a protease-responsive sensor and tested our platform first in vitro by measuring the activity of enzymes involved in tumor growth and spread of cancers against our sensors. We then moved in vivo to a mouse model of colorectal cancer (Fig. 1). We were encouraged by the first proof-of-concept disease model experiment, where we injected our sensors, collected urine 1 h later, and ran our colorimetric assay. Urine from tumor-bearing mice exhibited a strong blue color that was visible to the naked eye, whereas urine from healthy control mice had no color change, indicating tumor-specific renal clearance of catalytic nanoparticles.
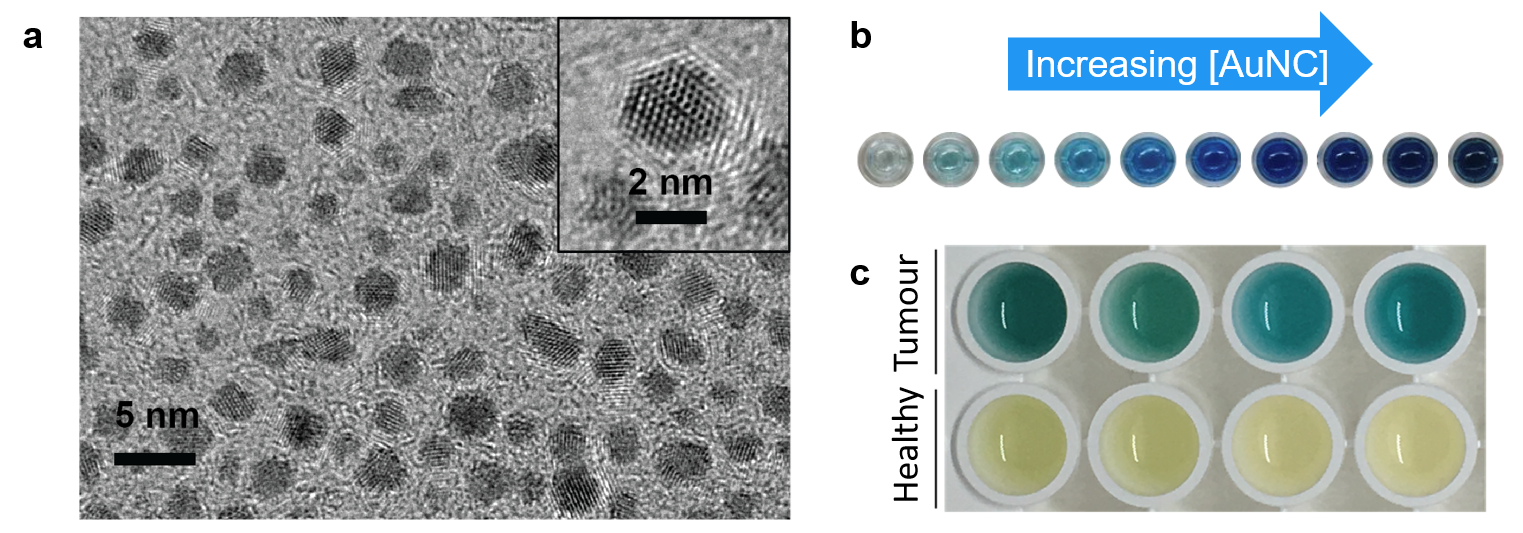
Figure 1. (a) Transmission electron micrograph image of ultrasmall catalytic gold nanoclusters (AuNCs). (b) Picture of varying concentrations of AuNCs in the presence of a chromogenic substrate and hydrogen peroxide, where the intensity of the blue colored dye increases with increasing AuNC concentration. (c) Photograph of representative examples of colorimetric assay on urine samples from tumor-bearing (top) and healthy (bottom) mice injected with nanosensors.
We are now looking into how we can adapt this technology for detection of other diseases and thinking about how we can translate this technology beyond the lab, which will involve improving the sensitivity and specificity of the system. We’re excited to build on this international collaboration and continue to marry the expertise and creativity of researchers at MIT and Imperial. Sangeeta’s group is based in the Koch Institute for Integrative Cancer Research bringing together biologists, engineers, and physicians, and Molly’s group sits at the intersection of the Departments of Materials Science and Bioengineering – these interdisciplinary environments feel like a playground for designing creative engineering solutions to complex biological questions that are driven by a clinical need. Through this project we brought together two seemingly disparate tools to create a new technology that has potential for broad impact in point-of-care diagnostics.
Written by Ava Soleimany and Colleen Loynachan.
References:
1. Kwong, G. A. et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat. Biotechnol. 31, 63–70 (2013).
2. Kwon, E. J., Dudani, J. S. & Bhatia, S. N. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nat. Biomed. Eng. 1, 0054 (2017).
3. Buss, C. G., Dudani, J. S., Akana, R. T. K., Fleming, H. E. & Bhatia, S. N. Protease activity sensors noninvasively classify bacterial infections and antibiotic responses. EBioMedicine 0, (2018).
4. Loynachan, C. N. et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 12, 279–288 (2018).
5. Wood, C. S. et al. Taking connected mobile-health diagnostics of infectious diseases to the field. Nature 566, 467–474 (2019).
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in