Tumors’ Stealth Tactic: How Cancer Disables Immune Defenses from Within
Published in Cancer
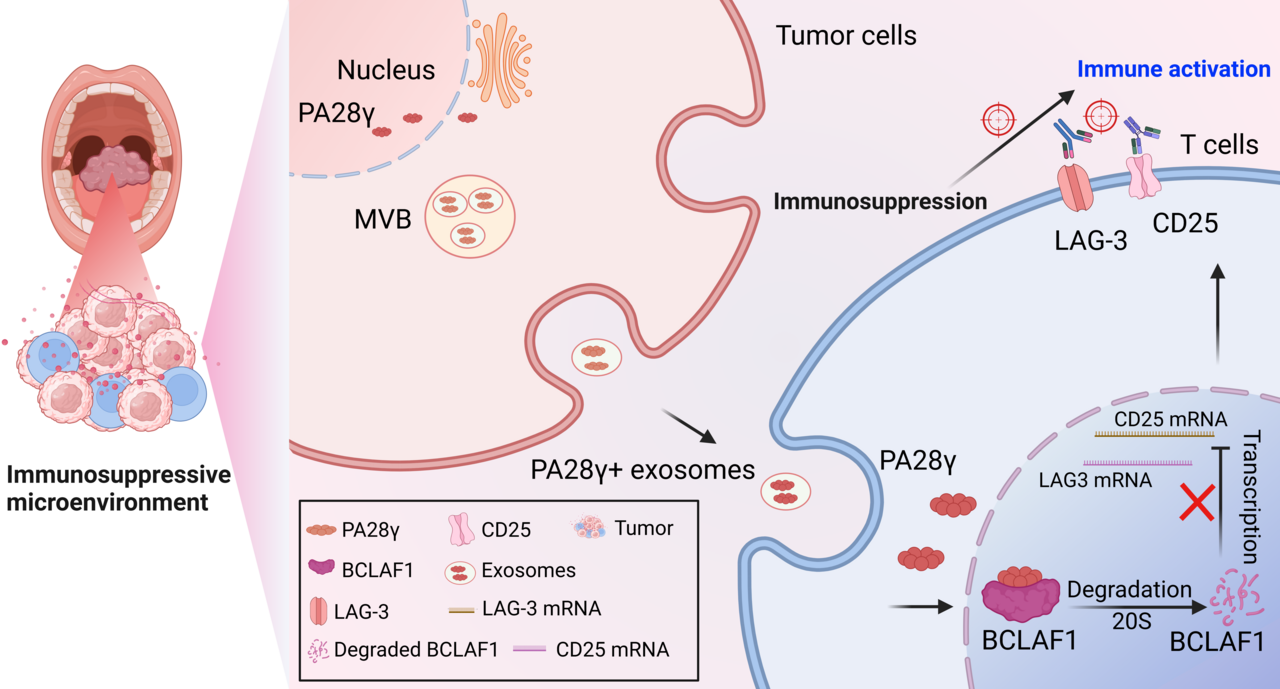
Exosomes: Cancer’s Trojan Horses
Exosomes are nano-sized vesicles that shuttle signals between cells. We discovered HNSCC tumors overpack these vesicles with PA28γ, a protein known to fuel cancer growth but little linked to tumor immune evasion. Using patient-derived models and advanced imaging, we confirmed PA28γ is selectively loaded into tumor exosomes and released into the tumor microenvironment—with clear evidence in clinical HNSCC samples.
How Tumor Exosomes Sabotage T Cells
When T cells internalize PA28γ-loaded exosomes, they undergo a dramatic transformation: Loss of function: They lose their ability to kill cancer. Exhaustion markers: CD25 and LAG-3 levels surge, signaling dysfunction. In mice lacking PA28γ, tumors overexpressing PA28γ grew faster and contained T cells with detectable exosomal PA28γ cargo. Conversely, blocking PA28γ restored T-cell activity and slowed tumor growth, proving its role in creating an immunosuppressive environment.
Mechanistic insight: PA28γ targets BCLAF1 for degradation
Mechanistic studies uncovered a direct interaction between PA28γ and BCLAF1 (BCL2-associated transcription factor 1) within T-cell nuclei. PA28γ promoted proteasomal degradation of BCLAF1, a key transcriptional regulator that maintains T-cell activation. Loss of BCLAF1 led to the derepression of CD25 and LAG-3 transcription, locking T cells into a dysfunctional, exhausted state. In essence, PA28γ acts as a molecular saboteur -infiltrating T cells via exosomes and dismantling their transcriptional machinery from within.
New Hope: Dual Checkpoint Blockade
This mechanism offers a promising target. In preclinical models, tumors with high PA28γ levels responded better to dual blockade of CD25 and LAG-3, suggesting a novel combination therapy could reverse T-cell exhaustion.
Clinical Evidence: PA28γ as a Biomarker
Analysis of 98 HNSCC patients showed: high PA28γ expression correlated strongly with CD25+ and LAG-3+T-cell infiltration, as well as with poorer overall survival. Serum PA28γ increased with tumor stage, hinting at its potential as a non-invasive diagnostic tool.
From protein degradation to immune evasion
Our work identifies a new “PA28γ–BCLAF1–CD25/LAG-3” signaling axis as a driver of T-cell exhaustion in HNSCC. Unlike classical immune escape mechanisms that rely on membrane checkpoint interactions (e.g., PD-1/PD-L1), PA28γ exerts its effects intracellularly, rewiring transcriptional programs to silence T-cell activity. By linking the fields of proteostasis, exosome biology, and tumor immunology, this study provides a conceptual advance in understanding immune evasion.
What’s Next?
PA28γ’s enzymatic nature makes it a druggable target. Future work will explore: Small-molecule inhibitors to block PA28γ activity or exosomal release. Combining PA28γ targeting with existing immunotherapies to enhance efficacy. This discovery isn’t just a scientific advance—it’s a reminder that cancer doesn’t hide; it dismantles. By understanding how tumors weaponize exosomes, we’re one step closer to turning the tide in immunotherapy-resistant cancers.
Stay tuned: The future of cancer treatment may lie in stopping the saboteurs before they strike.
Follow the Topic
-
Cellular & Molecular Immunology

A monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, covering both basic immunology research and clinical applications.
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcement



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Interesting issue,thanks for sharing