Understanding Electrolytes and Interface Chemistry for Sustainable Nonaqueous Metal–CO2 Batteries
A cross-continental team from North China Electric Power University, TU Ilmenau and the University of Central Florida, led by professors Huajun Tian, Yong Lei and Yang Yang, has released a 34-page strategic roadmap in Nano-Micro Letters that demystifies how electrolytes and interfaces decide the fate of non-aqueous metal–CO2 batteries. Their review, “Understanding Electrolytes and Interface Chemistry,” distills ten years of explosive progress and maps the next five toward scalable, high-density storage that converts greenhouse gas into grid-level energy.
Why Electrolytes Matter
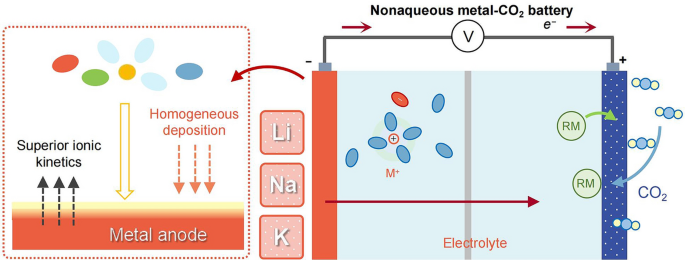
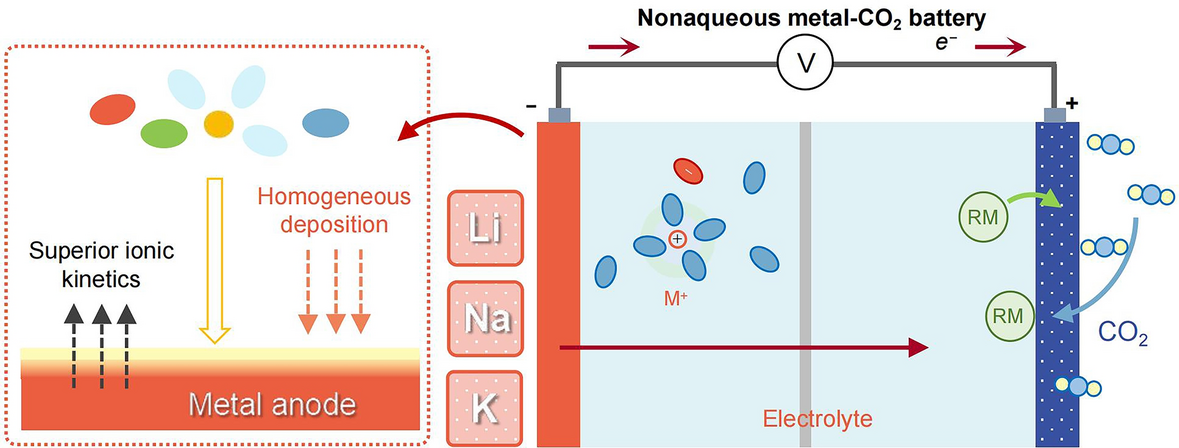
Carbon-to-Current Conversion: A Li–CO2 cell can, in theory, deliver 1,876 Wh kg-1—five-fold today’s Li-ion—by fixing CO2 as Li2CO3 during discharge. However, the electrolyte controls every step: CO2 solubility, nucleation of carbonates and the critical over-potential for their reversible decomposition.
Interface Stability Gap: Early systems lose 20 % capacity in <50 cycles because carbonate deposits rupture the solid-electrolyte interphase (SEI) on lithium, sodium or potassium metal. The review shows how targeted electrolyte chemistry now pushes this barrier past 400 stable cycles.
Engineering the Electrolyte–Interface Duo
Liquid Electrolytes Re-engineered: Adding 1 M LiPF6 to LiTFSI/TEGDME cuts desolvation energy by 30 % and forms a LiF-rich SEI that suppresses dendrites, extending cell life to 441 cycles at 500 mA g-1.
Redox-Mediator Boost: 10 mM I2/I3- shuttles drop the Li2CO3 decomposition over-potential from 4.5 V to 3.85 V, unlocking 95 % round-trip energy efficiency at 100 mA g-1.
Ionic-Liquid Hybrid: An IL@MOF electrolyte widens the electrochemical window to 4.7 V and retains 60 % capacity at −60 °C, enabling Martian-atmosphere operation.
Solid-State Leap: A PVDF-HFP/Na3Zr2Si2PO12 composite solid electrolyte delivers 28,830 mAh g-1 with 1.4 V hysteresis and survives 2000 h at 150 °C without leakage or volatilization.
Characterizing the Interface in Action
Operando XPS Tracking: During the first five cycles, Li2CO3 content in the SEI drops 70 % while LiF remains constant, confirming that fluorinated additives create a self-healing, ion-conductive but electronically insulating barrier.
Environmental TEM Snapshots: In K–CO2 nanobatteries, the same SEI stabilizes hollow K2CO3 spheres that swell and contract reversibly, visualizing the “breathing” mechanism critical for long life.
Future Outlook
Dual-Electrolyte Architectures: Bilayer polymer–ceramic electrolytes promise 5 V stability and continuous ion paths, targeting >500 Wh kg-1 packs.
AI-Guided Additive Screening: Machine-learning models trained on 12,000 electrolyte formulations predict optimal Lewis acid–base pairs to cut experimental cycles from weeks to days.
Temperature-Resilient Designs: Local high-concentration electrolytes with low-polarity diluents are poised to operate from −80 °C to +120 °C, matching desert nights to engine bays.
By translating molecular-level electrolyte design rules into macroscopic battery metrics, the Yang team turns CO2 from a liability into a limitless energy carrier, accelerating the convergence of carbon neutrality and next-generation storage.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in