
Carbon, a fundamental building block of life, exhibits diverse material forms each possessing unique properties. From the three-dimensional diamond to the layered graphene, carbon's versatility in forming different allotropes continues to captivate scientists, with new forms being discovered. The very recent synthesis of a novel two-dimensional carbon allotrope, graphullerene, consisting of C60 molecules, opens another chapter in carbon materials. We have studied this semiconducting material that challenges traditional notions about carbon's electronic behavior, by first-principles simulations. We explored the structure, properties, and potential applications of graphullerene, shedding light on this fascinating addition to the carbon family.
Ab initio calculations reveal that graphullerene's structure comprises of strained C60 molecules, interconnected in a hexagonal network (see Fig. 1) and resulting in a large mechanical strain energy. Each C60 molecule forms a total of eight chemical bonds with six neighbors, which balance with the strain energy to create a mechanically stable, yet thermodynamically meta-stable structure. The resulting graphullerene structure exhibits both sp2 and sp3 hybridization states, presenting a unique combination of electronic configurations not observed in other carbon allotropes.
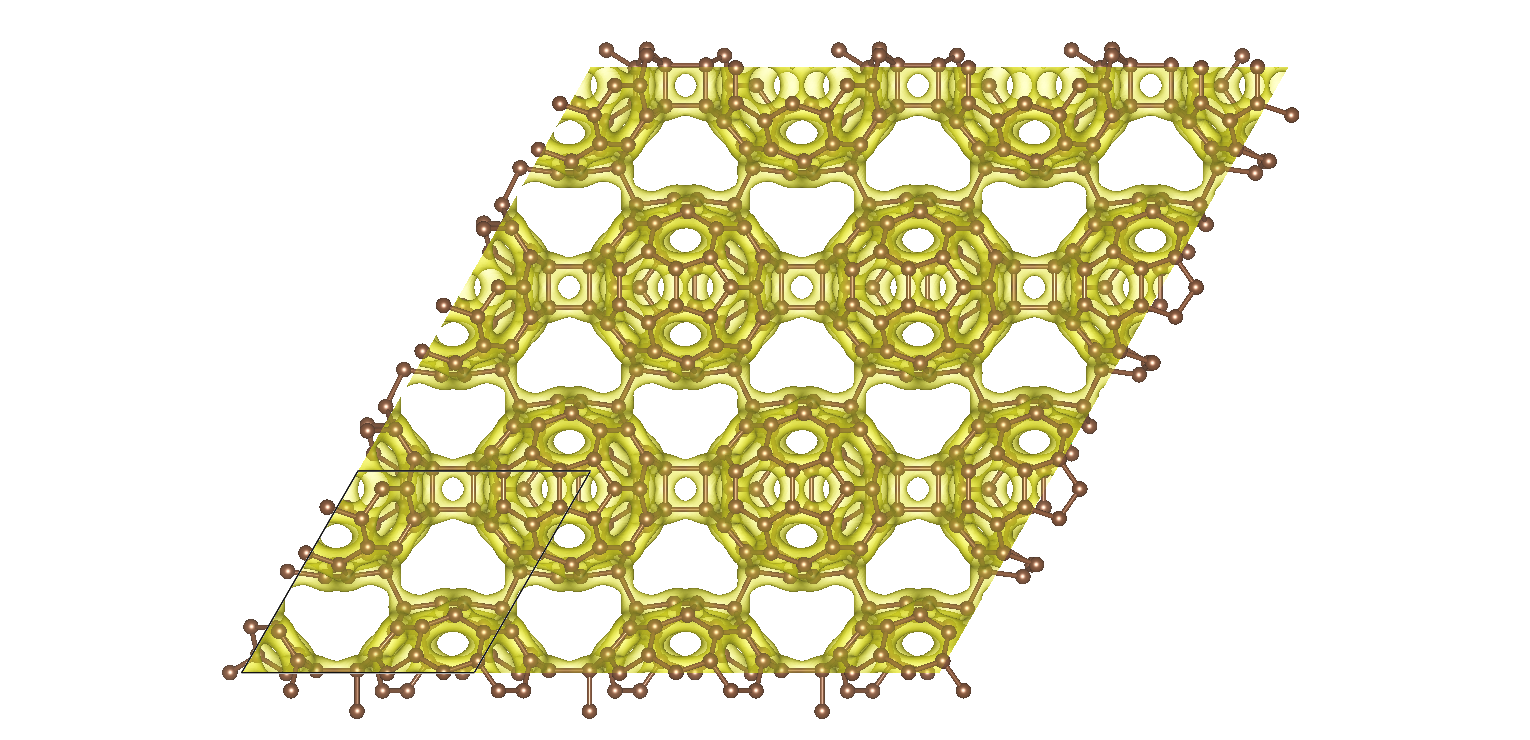
Fig. 1 Two-dimensional projection of the atomic structure of graphullerene together with a high electron density iso-surface. The black lines indicate a primitive unit cell.
Graphullerene was synthesized initially as (C60Mg4)n, and transformed into pure (C60)n by removal of the Mg atoms. Interestingly, the inclusion of Mg atoms in the crystal strengthens its cohesion energy, making it thermodynamically stable. The role of Mg atoms in synthesizing graphullerene is crucial. Introducing Mg atoms forms additional C-Mg bonds, transforming the cohesion energy from negative to positive values. The bridging effect of Mg atoms enhances the stability of the structure, confirming the experimental synthesis process where Mg atoms are initially incorporated and later removed.
Phonon spectrum calculations confirmed the dynamical stability of graphullerene, unveiling its in- and out-of-plane sound velocities. Symmetry breaking of the C60 molecules introduces a distribution of bond lengths and creates vibrational modes, serving as a distinctive signature of graphullerene. This aligns with experimental observations of Raman spectra, which showed the splitting of some vibrational modes.
In the absence of Mg, graphullerene emerges as a pure carbon semiconductor with an indirect band gap of 0.7 eV. This discovery positions graphullerene as the first two-dimensional pure carbon semiconductor.
Graphullerene's unique combination of structural and electronic properties opens up exciting possibilities for technological applications. As a two-dimensional semiconductor, it expands the toolkit of materials for various applications in electronics and beyond. The incorporation of Mg atoms not only stabilizes the structure but also influences the electronic band gap, offering tunability for specific applications.
In conclusion, graphullerene is a testament to the versatility of carbon, joining the ranks of diamond, graphite, and graphene. This two-dimensional semiconductor, with its unique combination of strained C60 molecules and the stabilizing influence of Mg atoms, represents a promising avenue for future research and technological advancements. As we continue to unravel the mysteries of graphullerene, it may soon take its place in the growing family of carbon-based materials, contributing to the ever-expanding realm of materials science.
Follow the Topic
-
npj Computational Materials

This journal publishes high-quality research papers that apply computational approaches for the design of new materials, and for enhancing our understanding of existing ones.
Related Collections
With Collections, you can get published faster and increase your visibility.
Computational Catalysis
Publishing Model: Open Access
Deadline: Mar 31, 2026
Recent Advances in Active Matter
Publishing Model: Open Access
Deadline: Sep 01, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in