Water’s Hidden Role: How Microenvironments Shape CO₂-to-Methane Conversion on Copper Catalysts
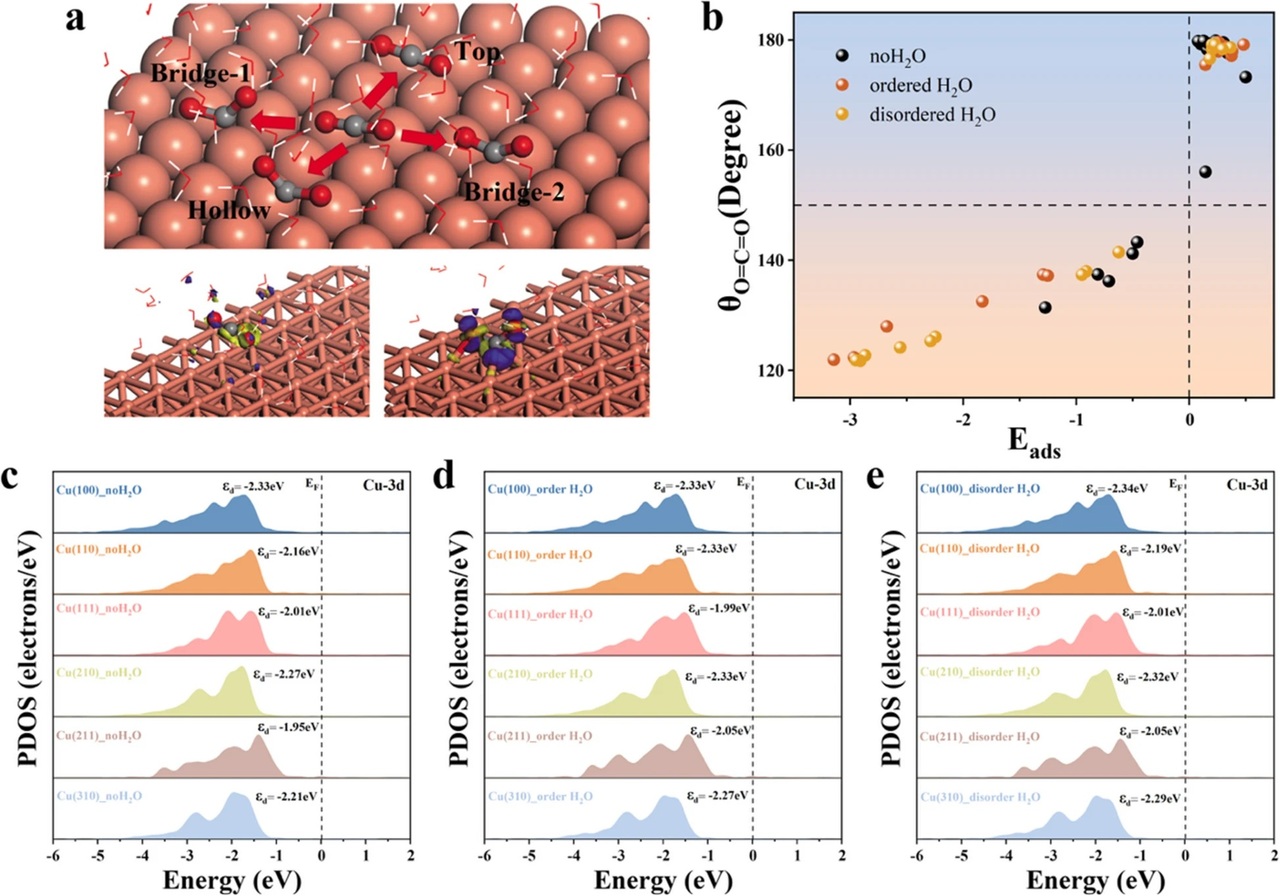
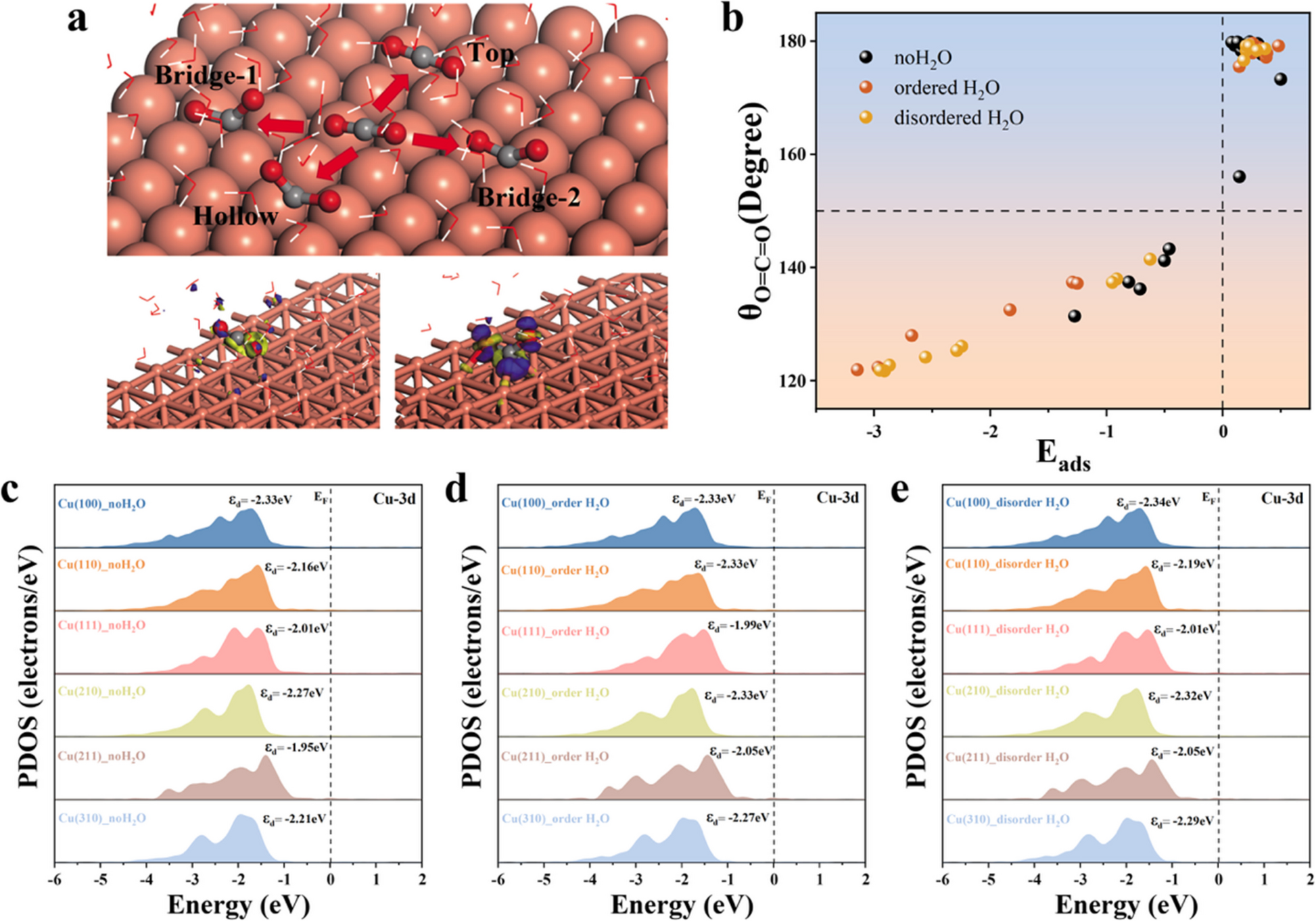
We are excited to share our latest research published in Catal, where we explore how the microscopic structure of water influences the electrochemical reduction of carbon dioxide (CO₂) to methane (CH₄) on copper surfaces.
This study addresses a critical gap in understanding the interplay between catalyst surface structure and solvation effects. Using density functional theory (DFT), we systematically investigated six copper crystal facets under three distinct aqueous conditions—dry, locally ordered water, and disordered water environments.
🔍 Key Insights from Our Study
- Disordered water enhances activity: We found that disordered H₂O environments significantly lower energy barriers for CO₂ activation, especially at bridge sites on Cu(100), promoting the CO2RR process.
- Surface geometry is decisive: Bridge and hollow sites consistently outperform top sites in facilitating intermediate adsorption and reaction progression.
- Scaling laws remain robust: Despite changes in water structure, the linear scaling relationships between intermediate binding energies persist, indicating a fundamental constraint in catalyst design on copper surfaces.
- Solvation alone isn’t enough: Tuning water environments alone cannot overcome thermodynamic limitations. Synergistic strategies combining catalyst surface engineering (e.g., alloying, doping) with solvation design are essential.
- Efficient modeling approach: Our use of static ordered/disordered water models provides a computationally efficient framework for capturing solvation effects and theoretical references for future dynamic simulations.
This work provides theoretical guidance for designing copper-based catalysts with improved selectivity and efficiency for CO₂-to-CH₄ conversion, contributing to the advancement of carbon-neutral technologies.
“Our findings highlight the importance of integrating surface structure engineering with solvation microenvironment design to achieve targeted improvements in reaction kinetics and product selectivity of CO2RR.”
— Bolong Huang, Corresponding Author
Follow the Topic
-
Catal

Catal is an open access journal covering full spectrum of catalysis critical advances. From biocatalysts to heterogeneous catalysts, it integrates fundamental and applied sciences. Catal offers a primary platform for researchers and practitioners in the field.
Related Collections
With Collections, you can get published faster and increase your visibility.
Bio-Catalysis in Circular Bioeconomy and Green Chemistry
This collection emphasizes the role of bio-catalysis in advancing the circular bioeconomy, focusing on enzymatic transformations and eco-friendly processes that valorize renewable feedstocks. Contributions should highlight innovative applications of bio-catalysis in waste-to-value systems, biorefineries, and green chemical synthesis.
Catal invites research articles, reviews and reports on the topic of the development of enzymes, metabolic engineering, and integration of bio-catalysis into industrial processes, aiming to reduce dependency on fossil-based resources and promote sustainable practices.
Publishing Model: Open Access
Deadline: Mar 31, 2026
Nanocatalysis and Thermocatalysis in Precision Chemical Synthesis
This collection, hosted by Catal, highlights the intersection of nanocatalysis and thermocatalysis in precision chemical synthesis. It aims to disseminate cutting-edge research that drives innovation in catalytic materials, selective processes, and reaction pathways, fostering advancements in the production of fine chemicals and specialty compounds. Aligned with Catal's mission to prioritize impactful catalytic applications, this collection welcomes contributions from established and early-career researchers that advance both theoretical and applied catalysis.
The collection embraces the breadth of Catal’s coverage, including topics such as nanostructured catalysts, thermocatalytic processes, and advanced synthesis strategies. Contributions may explore catalytic mechanisms, computational modeling, or experimental breakthroughs, offering insights into scalable industrial applications and fundamental research. Articles types—original research, reviews, perspectives, and analyses—are all encouraged, ensuring a diverse platform for sharing high-impact advancements in catalysis.
Publishing Model: Open Access
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in