Why do pneumococcal cells stop dividing during transformation ?
Published in Microbiology

The paper in Nature Communications is here: http://rdcu.be/yUJP
Natural competence for transformation is a physiological state that enables bacteria to capture, internalize and integrate DNA from their environment into their genome by homologous recombination. Competence is a regulated property. With few exceptions, it develops transiently at a specific growth phase, which varies according to species. In Streptococcus pneumoniae, competence occurs during early exponential growth and lasts for a period of about 25 minutes, shorter than the generation time of a single cell. The finding that stress conditions threatening cell survival induce competence, together with the absence of an SOS system in S. pneumoniae, led Jean-Pierre Claverys* to propose that this bacterium could use competence as a substitute for SOS. In line with the cell division blockage observed during the SOS induction in response to DNA damage, Jean-Pierre also sensed a link between the development of competence and the cell cycle.
We quickly tested Jean-Pierre’s idea and demonstrated that he was right. Using time-lapse microscopy, we found that cell division was delayed in competent cells (Fig. 1). The measurement of morphological parameters of individual cells indicated that both the initiation of cell division and the final constriction of the cytokinetic ring were delayed. We finally identified ComM, a membrane protein induced during competence, as the factor that is necessary and sufficient for these alterations. ComM was previously described as the immunity protein involved in fratricide, the killing mechanism that can be used by competent cells to acquire DNA from non-competent pneumococci.
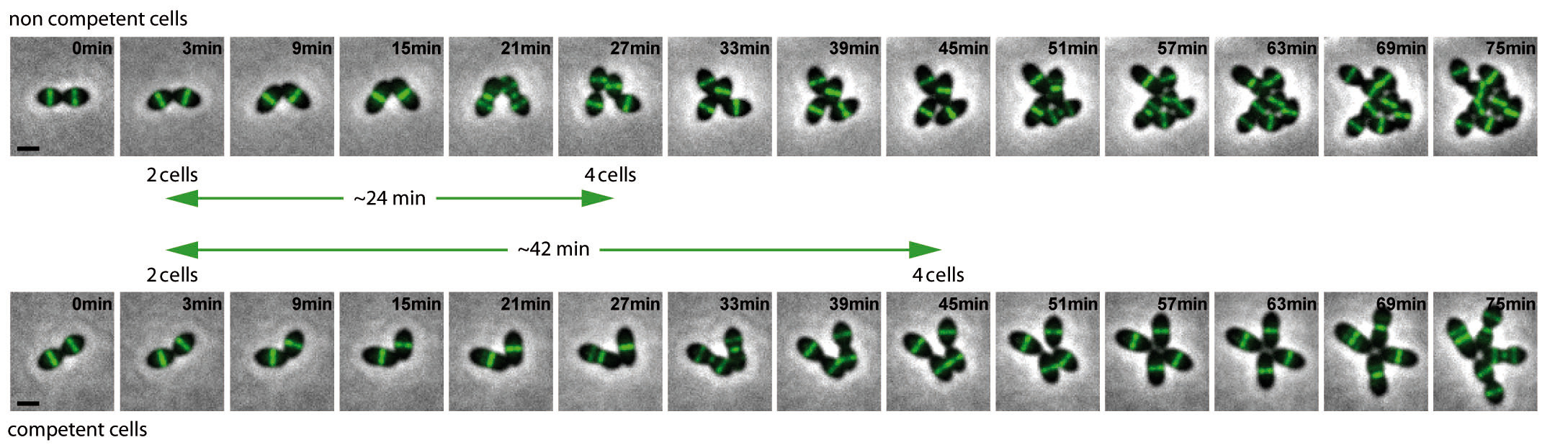
Figure 1: Still images from fluorescence time-lapse microscopy of cells producing a functional FtsZ-GFP fusion at 37°C.
At that point, we had discovered that competence interrupts cell cycle progression but we didn’t know why. This question really niggled at us: what could be the advantage of interrupting cell division during the transformation process? The cell division delay could just be the price to pay to protect competent cells from fratricide, but we wondered whether it could have another purpose. This was the beginning of a long list of false hypotheses… Here are some of the most promising ones that we tried:
We first verified whether the cell division delay was important for the localization of the transformation machinery, a large protein complex spanning the cytoplasmic membrane and the cell wall. We had previously shown that this machinery localizes at midcell in predivisionnal cells and at the future cell division sites in dividing cells; in other words at the division sites before cell constriction initiates. We performed fluorescence microscopy to localize different components of the transformation machinery, but the assembly and the stability of these proteins appeared unchanged in cells lacking ComM. We also measured the opening time of the DNA entry pore. For this, we developed a strategy in which we sequentially fed competent cells with donor DNA fragments carrying genes conferring resistance to different antibiotics and we selected for double transformants. Again, wild-type and comM mutant cells behaved the same.
We also wondered if the cell division delay had something to do with the presence of a polysaccharide capsule at the surface of pneumococcal cells. This idea was suggested by Sérgio Filipe when we met at an ASM conference. Sérgio had shown that capsule synthesis is coordinated with cell division. He had identified null mutants that were able to produce capsule attached to the cell surface but that was absent from the division site. His suggestion was that ComM could interfere with capsule synthesis during competence, resulting in the absence of polysaccharide at the division site, thereby facilitating the capture of exogenous DNA. We were really excited with this idea. We thus measured the transformation efficiency in several strains with different thickness of capsule layer and different concentrations of donor DNA, but we were never able to detect a difference between wild-type and comM mutants.
Finally, in view of the parallel between competence and stress response, we tested the possibility that the cell division delay preserves genome integrity by allowing cells enough time to complete transformation (Fig. 2). While it is generally assumed that the role of cell division inhibitors induced during the SOS response is to delay cytokinesis until after the genome has been repaired and fully replicated, this has never been proven. Here, we had a chance to test this using transformation assays. We knew that the transformation frequencies for a donor DNA carrying a single point mutation conferring resistance to an antibiotic were similar for strains containing or lacking ComM. We tried instead different donor DNA that could induce the formation of chromosomal rearrangements such as insertions and deletion of several kilobases, as well as inversions and large duplications that necessitate the formation of chromosome dimers as intermediates. Our reasoning was that these structures may require lengthy periods of time for their integration. With these new donor DNAs, we did observe 2 to 3-fold reductions in transformant frequencies of comM mutants. As Jean-Pierre used to say: “whatever the amount, if we halve our a salaries, we will certainly find this reduction as significant!”. Nonetheless, to reveal and emphasize the role of ComM during transformation, we eventually combined the comM mutation with mutations of genes involved in the resolution of chromosome dimers. Our results confirmed the importance of the cell division delay in maintaining genome integrity when the chromosome is engaged in recombination events during transformation.
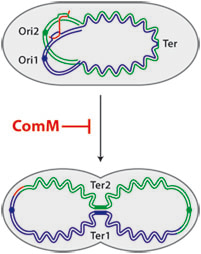
Figure 2: ComM preserves genome integrity during transformation by postponing cell division
The paper in Nature Communications is here: http://go.nature.com/2zVAMwa
Matthieu Bergé, Patrice Polard and Nathalie Campo
*Jean-Pierre Claverys headed the “Pneumococcal Tranformation” team (Toulouse, France) for more than 40 years. J.P. Claverys and P. Polard fused their groups in 2010 and co-directed the team until the retirement of J.P. Claverys in 2014.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in