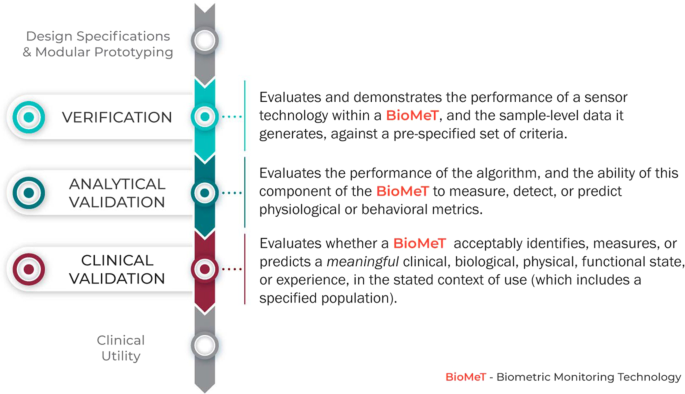
Why V3 (verification, analytical validation, and clinical validation)?
Published in Healthcare & Nursing
The development of Biometric Monitoring Technologies (BioMeTs) is helping drive the rapid growth of digital medicine. We define BioMeTs as connected digital medicine tools that process data captured by mobile sensors and use algorithms to generate measures of behavioral and/or physiological function. These technologies are often marketed as “validated”, but what does that actually mean? Currently, there is no systemic, evidence-based evaluation framework for these technologies.
In this article, a multi-disciplinary group of experts from the Digital Medicine Society (DiMe) propose and describe a three-component framework intended to provide a foundational evaluation framework of BioMeTs. This framework includes 1) verification, 2) analytical validation, and 3) clinical validation (Figure 1).
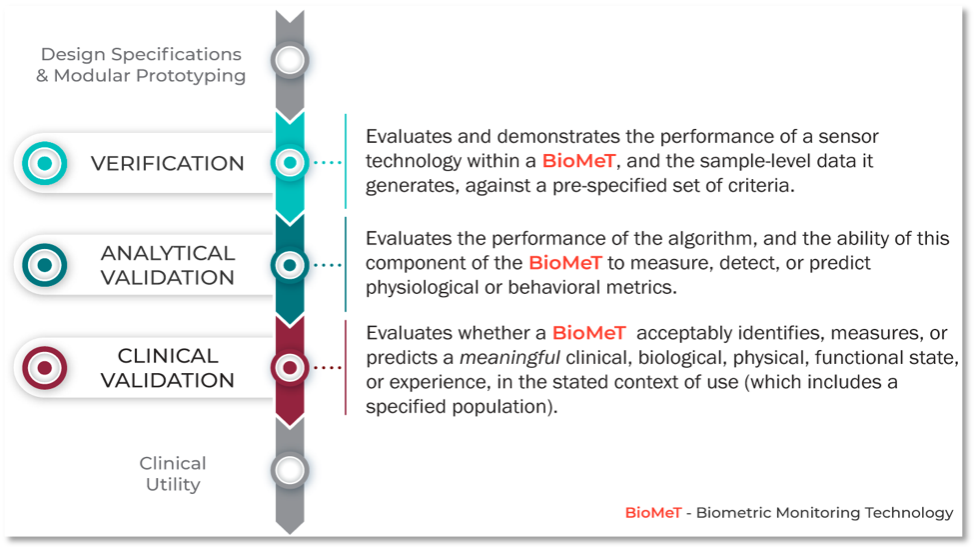
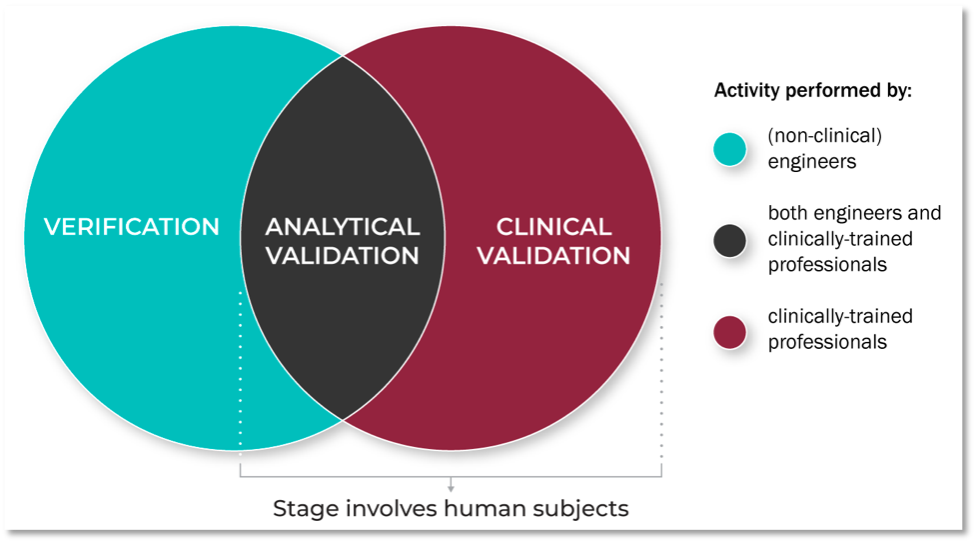
Figure 1. What is the V3 Framework?
For decades, two terms - verification and validation - have been used to describe critical components of successful quality management systems. The software, hardware, and regulatory industries have long histories of verification and validation as part of their quality management systems, which lay out specific requirements that must be met in order to comply with standards.
Although traditional validation for a software or hardware product confirms that the end product accurately measures what it claims to measure, BioMeT-derived metrics from digital tools must also be clinically useful to a defined population. As such, and to harmonize this framework with the language used to describe traditional clinical assessments, we distinguish between analytical validation and clinical validation.
This novel three-component V3 framework intentionally combines established practices from both software and clinical development. The definitions for verification, analytical validation, and clinical validation were derived from guidance documents, historical, and current frameworks ranging from 2002 to 2018. This paper seeks to also provide examples of using verification, analytical validation, and clinical validation in practice in addition to providing example use cases to illustrate the V3 framework. The V3 Process in Practice is shown in Figure 2.
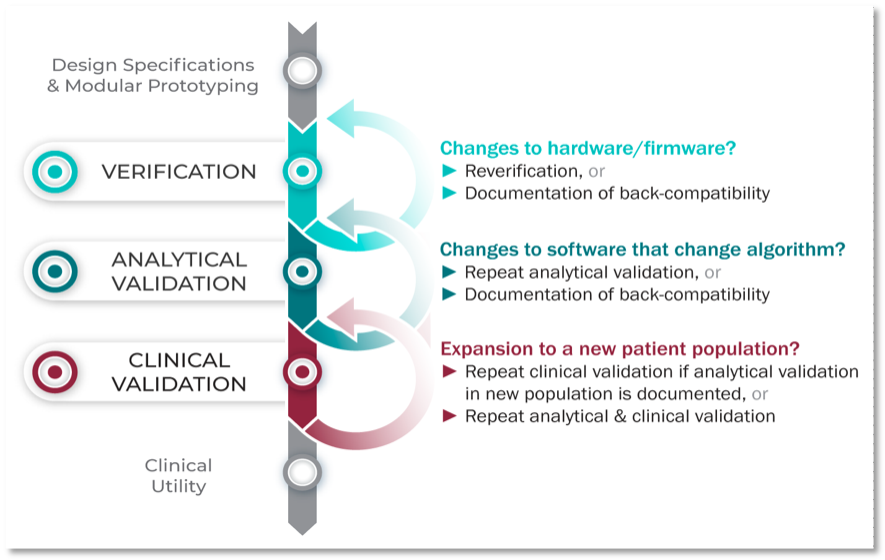
Figure 2. How can I apply the V3 Framework in practice?
Digital medicine is an interdisciplinary field, drawing together stakeholders with expertise in engineering, manufacturing, clinical science, data science, biostatistics, regulatory considerations, ethics, patient advocacy, and healthcare policy, to name just a few. The V3 processes are conducted by experts across disciplines and domains, as shown in Figure 3. While this diversity is undoubtedly valuable, it can lead to confusion regarding terminology and best practices. Establishing a common, unifying language to describe evaluation standards for BioMeTs is critical to streamline trustworthy product development and regulatory oversight. In this new era of digital medicine, we need a common lexicon containing consensus definitions to support a broad, interdisciplinary approach and advance the field.
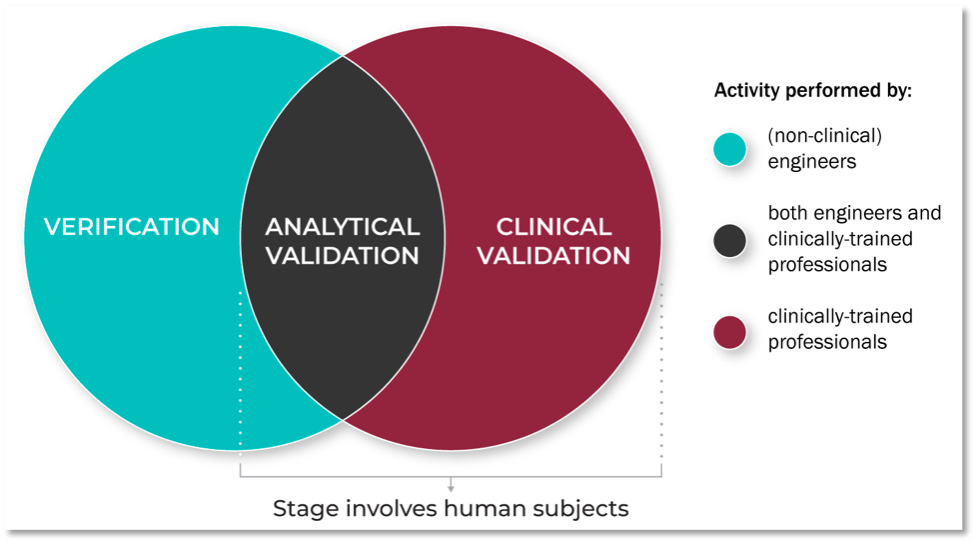
Figure 3. Who is responsible for performing V3?
Trust is required to adopt any new technology, and the V3 process for BioMeTs builds an evidence base to increase that level of trust. Professional societies like DiMe are a collaborative hub for innovation in this area. Our hope is that the V3 framework and definitions continue to evolve to reflect the technologies that they serve.
Follow the Topic
-
npj Digital Medicine

An online open-access journal dedicated to publishing research in all aspects of digital medicine, including the clinical application and implementation of digital and mobile technologies, virtual healthcare, and novel applications of artificial intelligence and informatics.
Related Collections
With Collections, you can get published faster and increase your visibility.
Evaluating the Real-World Clinical Performance of AI
Publishing Model: Open Access
Deadline: Jun 03, 2026
Impact of Agentic AI on Care Delivery
Publishing Model: Open Access
Deadline: Jul 12, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in