A Breath of Fresh Air: New Protein Inhibitors Offer Hope for Lung Fibrosis Patients
Published in Biomedical Research
A Breath of Fresh Air: New Protein Inhibitors Offer Hope for Lung Fibrosis Patients
Idiopathic Pulmonary Fibrosis (IPF) is a debilitating lung disease that affects millions worldwide. If you or a loved one has been diagnosed with IPF, you know how limited the treatment options are. Current therapies often offer little more than a temporary reprieve from the symptoms, leaving patients and their families searching for more effective solutions.
Our latest research paper introduces a groundbreaking approach to treating IPF using highly selective protein inhibitors. We created these inhibitors from scratch using computational protein design, a rapidly maturing field of research that allows for the creation of new medicines with custom properties. This approach allowed us to overcome one of the biggest challenges in drug development: creating molecules that are highly selective for their intended targets.
In the realm of drug development, achieving high selectivity is particularly challenging for integrins, which are a family of cell surface receptors involved in various diseases, including cancer and fibrosis. Our research focused on two specific integrins, αvβ6 and αvβ8, which are known to play a role in IPF and cancer. Using advanced computational methods, we created small proteins that selectively “block” these integrins, effectively turning off their harmful effects.
One of the standout features of our protein inhibitors is their remarkable stability, which allows them to be sprayed as a mist — or nebulized — and inhaled directly into the lungs. We showed this was possible in our paper, and we believe it could be a game-changer for patients. Traditional biologics like antibodies often degrade when nebulized, but our inhibitors remain stable, making inhalation delivery possible.
When it comes to lung diseases, inhaled medicines aren’t just convenient — they also target the treatment to where it is needed most. In vivo studies confirmed that our new inhibitors are rapidly cleared from the bloodstream but have a longer half-life in the lungs, making them ideal for pulmonary delivery.
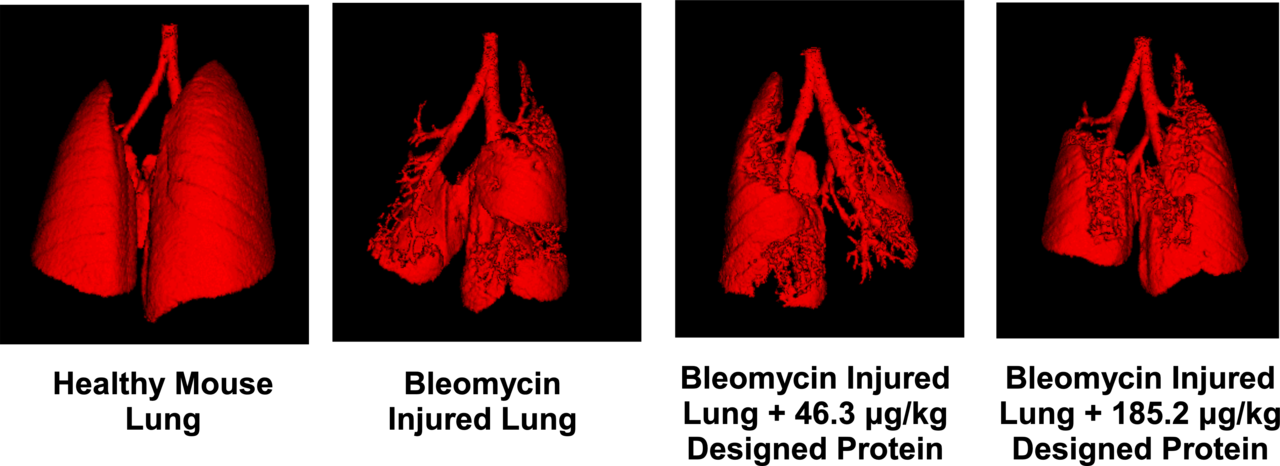
When tested in mouse models of IPF, we observed significant improvements in lung health and function, including reduced fibrotic progression and improved respiratory mechanics. These results were notably better than those achieved with existing treatments.
Our research also extends to human lung organoids, where we observed a significant reduction in pro-fibrotic markers after treatment with our inhibitors (Figure 1). This adds another layer of evidence supporting their potential effectiveness in treating IPF in people.
Moreover, our inhibitors have shown promise in combating progressive respiratory diseases associated with current and future coronavirus infections. This opens up new avenues for their application beyond IPF.
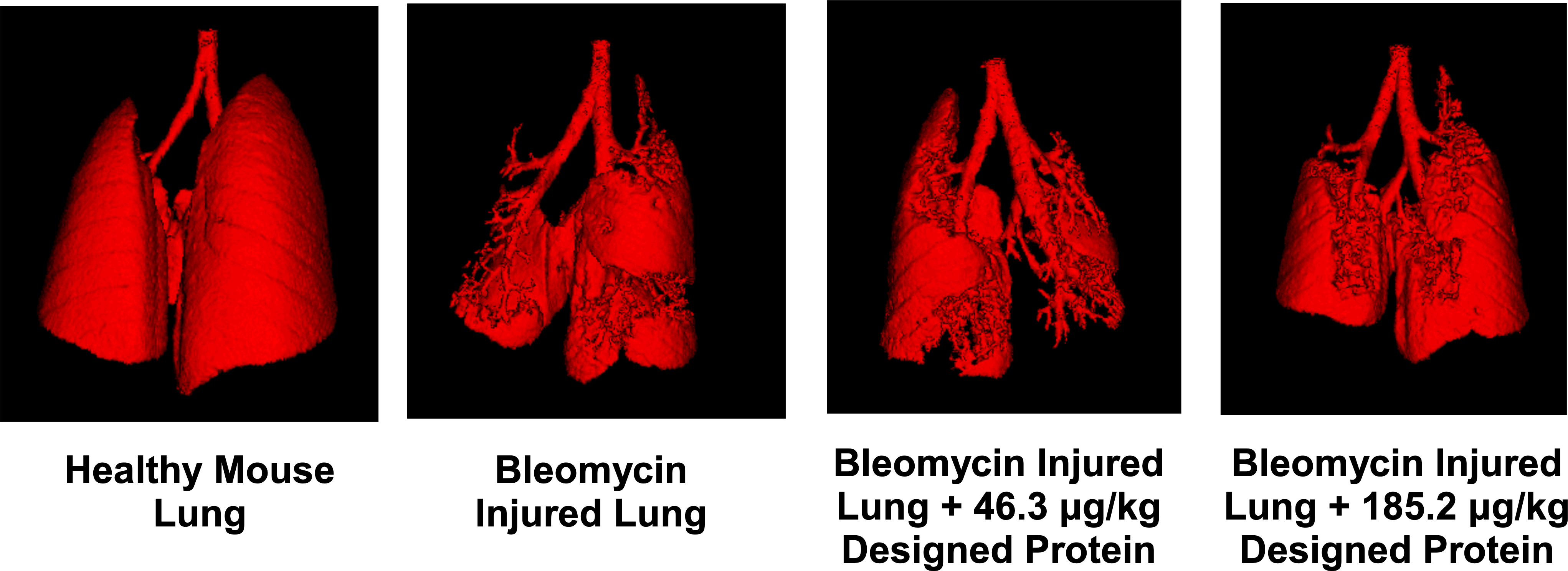
Figure 1: μCT scan shows clear improvement in overall lung architecture and a reduction of fibrotic burden in an animal model of IPF when treated with the designed binder.
Our work represents a significant step forward in developing targeted therapies for IPF and potentially other respiratory diseases. The ability to deliver these inhibitors via inhalation could revolutionize treatment options, offering patients a more effective and less invasive alternative to current therapies.
The next steps in our research include optimizing the inhibitors further and preparing for clinical trials. We are optimistic that our work will pave the way for new, life-changing treatments for those suffering from IPF and related conditions, but as always, clinical development of new medicines takes time and is never guaranteed to succeed.
For a more in-depth understanding, please refer to our research paper titled "De novo design of highly selective miniprotein inhibitors of integrins αvβ6 and αvβ8" (doi:10.1038/s41467-023-41272-z).
This research was led by the Institute for Protein Design at the University of Washington School of Medicine and included key collaborators from the Fred Hutchinson Cancer Center, Brigham Young University, and Harvard Medical School.
Contributed by Ian Haydon, Institute for Protein Design.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in