A bumpy road
Published in Materials

Understanding mechanisms arising from phenomena six orders of magnitudes in length scale below what we can see, is challenging. We need to stitch together the “truth” from the different snapshots that we get using various measurement methods. Of course, all these methods will have their advantages and limitations, which is why they are all needed in order for a complete mental picture to be formed.
Material science is at the juncture of chemistry, physics and engineering, and all three disciplines were important when understanding the behavior of rough particle colloidal gels. Colloidal gels are formed, when attractive interactions are induced in a suspension of colloidal particles (which typically are in the size range 10 nm - 10 µm in diameter). The particles then form a spanning network which macroscopically forms a gel. This material is visco-elastic, meaning that it has liquid-like and solid-like characteristics, where the particle network contributes to the elasticity and the suspending medium contributes to the viscous behavior. By tuning the particle material, shape, topography as well as the suspending media properties, the network properties will change, which in turn also change the macroscopic properties. These are only a few examples of the “knobs to turn” so we can engineer a gel with targeted properties. In this work, the initial idea was to render a gel tougher by introducing surface topography to the building blocks of the gel. The thinking was that if the particles would behave similar to a jammed assembly of gears, they would interlock and resist to rolling when exposed to stresses. With this mental image in mind, the next step was to realize these particles in a reproducible manner, make the gel and find the right experiments to prove the hypothesis, that smooth and rough particles will behave different when exposed to shear stresses.
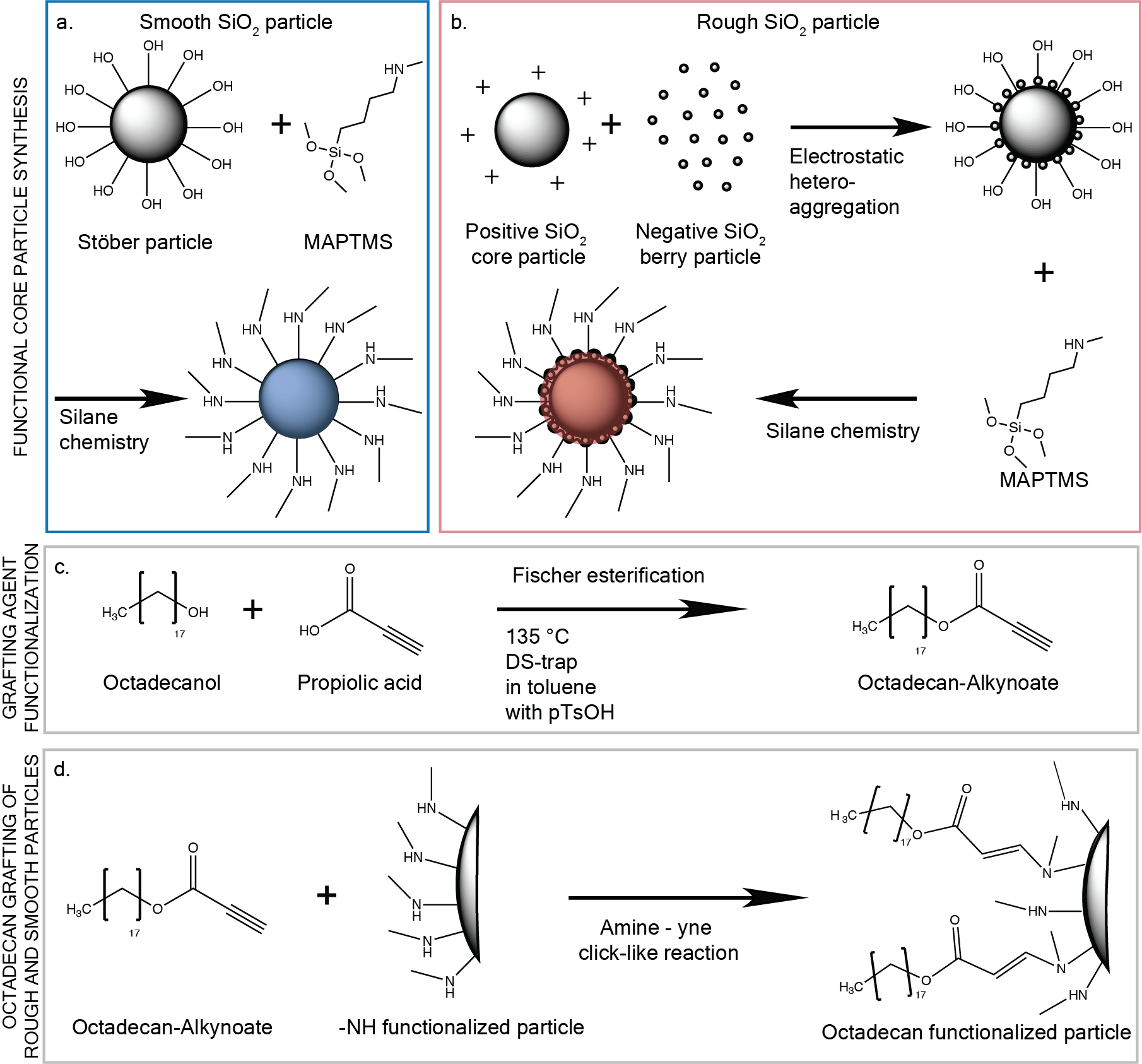
Chemistry is a very powerful tool when designing nanoparticles. Often, particles can be made of smaller molecules that naturally assemble to smooth spherical structures, which is dictated by surface energy. By understanding the particle properties, the features can be utilized to engineer more complex geometries, a kind of “nano-lego”. The synthesis of the rough particles was built on a previously developed heterocoagulation method, which uses electrostatic effects to add small particles to the surface of large particles: as the particles are negatively charged in water, adding a positively charged molecule to the suspension will make the molecule adsorb to the particle surface and make it positive. If negatively charged smaller particles are then added to this suspension, they will in turn adsorb to the positively charged cores. This is how the rough particles were synthesized in this work.
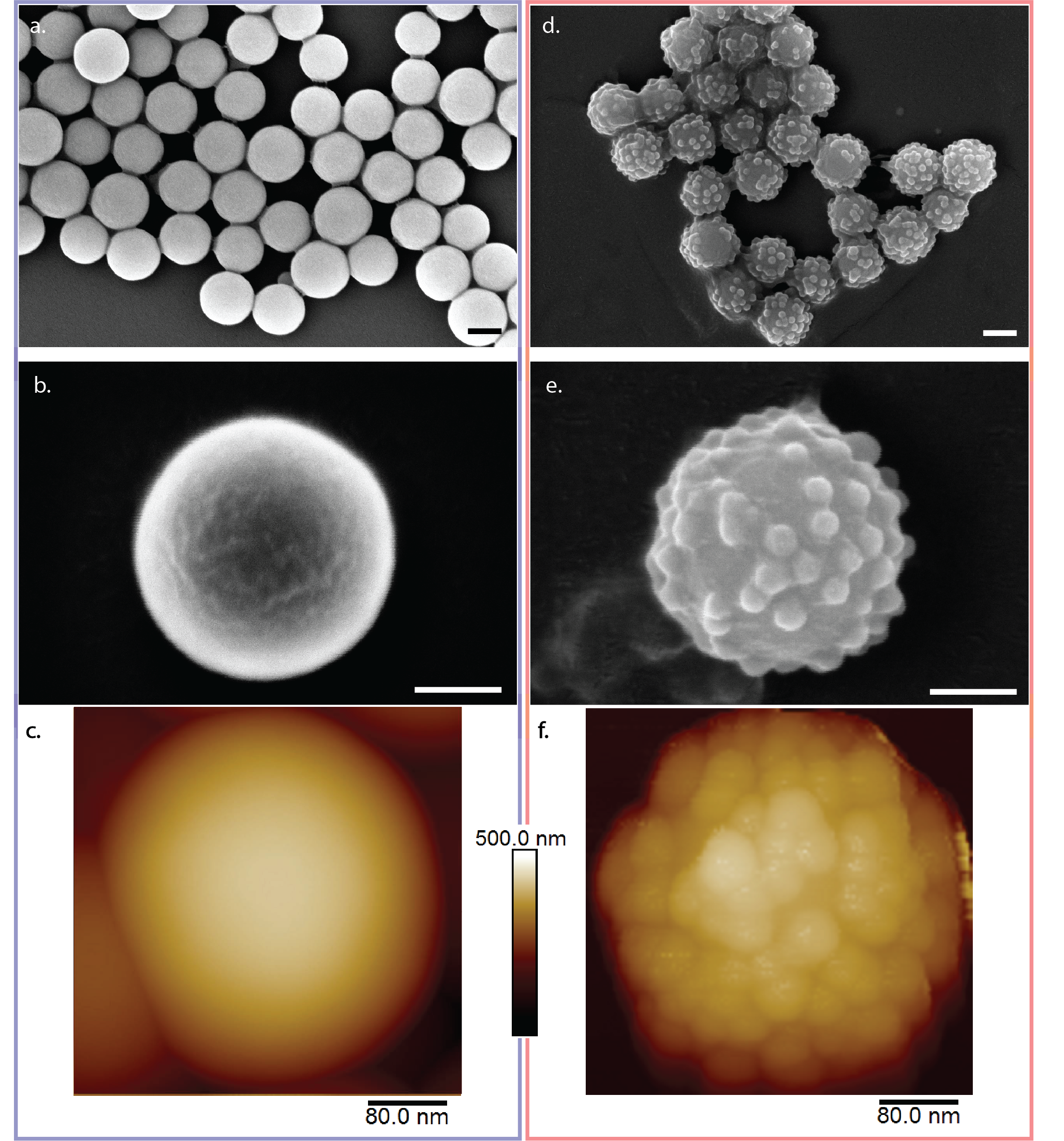
In a next step, the particles had to be assembled into a gel, which means that attractive interactions must be induced between the particles. This can be done in many ways, however we had a few design requirements for the gel system: (i) as the measurements can be long, evaporation needed to be limited, therefore water was not a good suspending medium; (ii) the interaction forces had to be the same for the smooth and rough particle system in order to best compare the results of both; (iii) most importantly, the gelation needed to be reversible through an independent trigger in order to form a nascent gel inside the measurement cell. The latter was most important to the study because colloidal gels are highly thixotropic, meaning that their mechanical properties depend on their processing and shear history. Therefore, even using a syringe or pipette to load the gel into the measurement cell, would have biased the comparison of the rough and smooth primary particle gels. An external trigger that can be controlled in most measurement systems is the temperature, which is why we proceeded in looking for a gelation strategy, where we could use the above-mentioned rough particles in combination with thermal gelation. Usually, in colloidal systems, thermal gelation is achieved in grafting long molecules to the surface of the particles, depending on the temperature, these molecules will interact differently with the suspending media and either have steric repulsion (liquid macroscopic behavior) or will induce the particles to aggregate into clusters (gel-like macroscopic behavior). Thinking back to our design requirements, if both types of particles (rough and smooth) are coated with the same molecule and to the same extent, the aggregation mechanism will be the same, meaning that the only difference in gel behavior will come from the bumpiness or the topography (while keeping the size of particle constant).
Thermoreversible colloidal gels are already widely used as model systems and for different applications, in water-based systems, the large molecule is usually PNIPAM. Grafting particles with PNIPAM is a multi-step process with a small yield. In this work, we used a well-known thermal gelling mechanism, where the particles are grafted with octadecyl (C18), which are suspended in tetradecane (C14). At warm temperatures, the octadecyl extends into the bulk phase and has steric interactions with surrounding particles, at low temperatures, the octadecyl crystalizes with the suspending media, inducing attractive interactions. Traditionally, the grafting is performed in a large batch, which is however very poorly controllable, inducing differences in the transition temperature and interparticle forces. Here, we developed a new and robust grafting method, where both types of particles, rough and smooth, can be grafted using click-like chemistry. For these particles to be thermoreversible, the grafting layer needs to be dense enough, otherwise there will not be the possibility of steric hindrance between the particles at high temperatures. As this click-like reaction is very efficient, it is a good method to achieve relatively high grafting densities with a grafting-to approach. A further advantage of this method is that batch sizes of 1g can be synthesized at the time, which is quite large for colloidal chemistry.
With this model system in hand, we could reproducibly probe the difference between rough and smooth colloidal gels by isolating the effect of the roughness and reducing synthesis effort. We found that adding surface roughness to the particles makes the gels tougher, meaning that they can resist to higher strains and stresses before they fluidize. Further, rough particle gels are self-healing, which means that they will reform to their original strength after shear stresses subside. This was shown to be beneficial in gel applications such as extrusion 3D printing and xerogel manufacturing.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in