A GIPR antagonist conjugated to GLP-1 analogs promotes weight loss with improved metabolic parameters in preclinical and phase I settings
Published in Biomedical Research and General & Internal Medicine
Our deepening recognition of obesity as a disease has intensified the call for more effective treatments for this pervasive health crisis. Worldwide, more than 650 million people are living with this common, serious and costly chronic disease, and nearly 2 billion individuals will be living with obesity by 2035 if rates continue to climb. The majority of current obesity treatments in the United States include FDA-approved glucagon-like peptide-1 (GLP-1) receptor agonists. These medicines, administered by injection daily or weekly, can promote weight loss. However, a growing body of real-world data suggests that currently available anti-obesity medicines can lead to treatment discontinuation and weight regain. Therefore, innovative treatments with improved tolerability and sustained weight loss are needed.
Pursuing a novel multi-specific molecule for obesity
Amgen has a strong scientific legacy, a long history in human genetics, and unique research capabilities in the area of obesity – all of which led to the eventual discovery and engineering of maridebart cafraglutide, or MariTide for short.
It began when our Cardiometabolic Disorders Discovery Research team at Amgen – the group I’m a part of – observed synergistic weight loss effects in mice on a high-fat diet when treated concurrently with a marketed GLP-1 analog and a mouse glucose-dependent insulinotropic polypeptide receptor (GIPR) blocking antibody. These findings sparked the idea to create a single molecule that could reproduce this effect, culminating in the creation of MariTide, characterized in this paper.
Our goal was to create a multi-specific molecule with the dual mechanism of GLP-1 agonism and GIPR antagonism that would have long-term stability as well as sustained efficacy in reducing weight and improving related metabolic parameters and comorbidities in humans. Multi-specific molecules have the potential to yield efficacy surpassing that of monotherapies. The strategic engineering of multi-specific molecules enables the creation of therapies that not only promise substantial efficacy and convenient dosing but also aim to be safer and more tolerable for humans. However, the study of multi-specific molecules adds a layer of complexity to our experiments, too, as the observed synergistic effect on weight loss may necessitate the close proximity of GLP-1 and GIPR receptors on cells. Further, the intricate variations in receptor expression among species contribute to the complexity of understanding the mechanism of action.
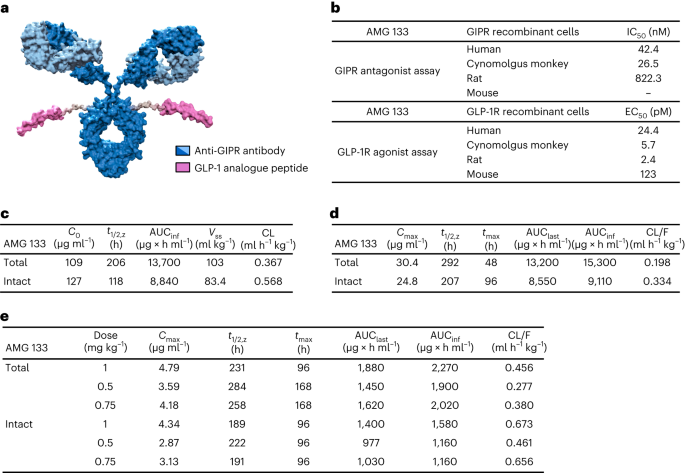
We invested considerable time and effort in the pursuit of identifying a GIPR antibody, resulting in the creation of various multi-specific molecules. Over a two-year period of screening, our team diligently assayed more than 150 different multi-specific molecules before identifying the one we thought would have the most promise and potential for impact – an antibody-peptide conjugate with two targets, named maridebart cafraglutide. The antibody component of MariTide inhibits GIPR and the GLP-1 peptide component activates the GLP-1 receptor.
Novel structure and MOA enhances its function
The distinct structure of MariTide and its unique mechanism of action play a crucial role in enhancing its function and effectiveness. The design of this molecule focuses on optimizing the combined impact of activating GLP-1 while inhibiting GIP receptors. A key benefit of MariTide is its ability to simultaneously bind to both targets. As described in the paper, in a monkey model of obesity, we observed a reduction in body weight, insulin and lipid levels with MariTide. In a mouse model of obesity, we observed significant, continual weight loss with a MariTide-like molecule and the mice stayed healthy.
At the molecular level, our findings suggest that when both receptors are in proximity, MariTide binds to GIPR and GLP-1R simultaneously, triggering early receptor internalization of GLP-1R and enhancing GLP-1-specific plasma and endosomal membrane cAMP production, which plays a role in various physiological processes, including metabolism. Because MariTide targets these two separate pathways associated with obesity, we hypothesize that it could result in rapid, substantial and sustained weight loss without the weight rebound often observed with other approved anti-obesity medications.
Our team displayed resilience and dedication in the screening phase, addressing factors such as the conjugation sites for GLP-1 peptides, designing and selecting specific GLP-1 peptides, and determining the suitable linker length to connect them to the GIPR antibody. These adjustments were beyond in vitro evaluation, necessitating testing every multi-specific molecule in our diverse animal models of obesity. The discovery and development of MariTide represents the culmination of the persistence and collective efforts of our obesity research team, a dynamic group that includes individuals specializing in in vitro work complemented by others focused on in vivo studies, all working collectively to achieve our shared goal of restoring health and saving lives.
Early clinical data supports potential of MariTide in treating obesity
Based on our preclinical findings that suggested synergistic effects on weight loss and improvement in other metabolic parameters with GIPR blockade and GLP-1 receptor agonism, as well as human genetic insights, Amgen initiated the clinical development of MariTide.
As described in the paper, the Phase 1 dosing study demonstrated rapid and sustained weight loss with MariTide. After just 12 weeks, study participants who received the highest dose (420 mg given subcutaneously every four weeks) achieved a 14.5% reduction in body weight. In contrast, placebo-treated study participants had a 1.5% increase in body weight. Furthermore, participants on the highest dose maintained substantial weight loss – 11.2% – for up to 150 days after the last dose. These results are the first to show this degree of durable weight loss from any investigational or approved anti-obesity medicine. Although study participants did not have diabetes, those treated with MariTide also experienced a decrease in fasting glucose from baseline, compared with no change with placebo, and a decrease in levels of high-sensitivity C-reactive protein, an inflammatory marker. Further, MariTide had an acceptable safety and tolerability profile, with gastrointestinal-related side effects the most common after the first dose and subsiding with subsequent doses.
Amgen is continuing to evaluate MariTide in a Phase 2 trial, which rapidly enrolled nearly 600 study participants with obesity with or without diabetes in 12 countries. This study is designed to find the appropriate therapeutic dose regimen for the Phase 3 clinical development program and to evaluate less frequent dosing schedules than those for existing anti-obesity treatments. We will study the Phase 2 data to assess whether the weight loss efficacy and improvements in metabolic and inflammatory biomarkers observed in preclinical models of obesity and in our Phase 1 trial will translate to a larger and more diverse group of people living with obesity.
Follow the Topic
-
Nature Metabolism

This journal publishes work from across all fields of metabolism research that significantly advances our understanding of metabolic and homeostatic processes in a cellular or broader physiological context, from fundamental cell biology to basic biomedical and translational research.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in