
Electrochemical conversion of CO2 and H2O to value-added chemical feedstocks and fuels, powered using renewable electricity, provides one route to tackling global energy and climate concerns.
Ethanol is a widely-used liquid fuel and fuel additive. It is particularly desirable because it is energy dense by both volume and mass, and because it takes advantage of the extensive existing infrastructure built for the distribution of fossil fuels. However, the selective production of ethanol from CO2 electroreduction has yet to be achieved: the Faradaic efficiency (FE) toward ethanol on state-of-art catalysts is still below 30%, and the overpotential is well above 1 V at appreciable production rates (current densities above 10 mA cm–2).
Prior electrocatalyst development has focused on tuning the binding strength of reaction intermediates such as CO. This approach is limited by the scaling relations, wherein the binding strength of one intermediate on the same site is correlated to that of the other intermediates along the pathway. This makes it infeasible to optimize each step along the pathway when a single class of active sites is exploited.
We pursued a catalyst design strategy that would break this compromise: we believed that – if we could increase the coverage of the key intermediate, CO, yet not interfere with its binding strength on an already optimized C–C coupling catalyst surface – we could boost the reaction rate of a key step and circumvent the compromise described in the scaling relations.
In our work, we report cooperative electrocatalysts in which we functionalized the metal catalyst with a family of molecular complexes (Figure 1). We took advantage of the superior ability of the molecular catalysts to convert CO2 to CO with near-unity FE.
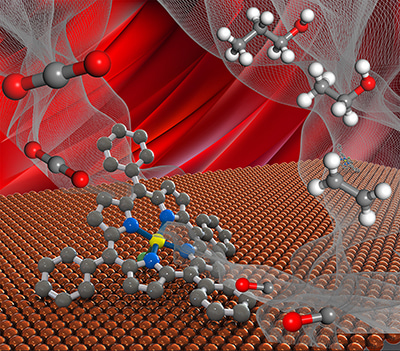
Figure 1. Schematic illustration of the molecule:metal interface catalyst.
We found that the resultant high concentration of CO at the molecule:metal interface promotes ethanol production from CO2. We achieved an electrochemical CO2-to-ethanol FE of 41% at a partial current density >100 mA cm–2 and an overpotential <1 V. This is by a factor of 1.4x the highest FE for ethanol reported using an electrocatalyst and operating above 10 mA cm–2.
We further utilized a set of tools, including density functional theory calculations, electrochemistry, in situ Raman and operando X-ray adsorption spectroscopies, to explore mechanisms underpinning the advance. We found that the locally created CO-rich environment at the interface facilitates C–C coupling and further favors the ethanol pathway over the competing ethylene pathway.
The findings presented in this work suggest new avenues to achieve electrocatalysis that bypass the traditional scaling relations. The new heteromaterials do so by tuning the intermediate enrichment at catalyst interfaces.
The full paper can be found here: https://www.nature.com/articles/s41929-019-0383-7.
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in