The paper in Nature Communications is here: http://go.nature.com/2FY3Oiq
The seeds of this work were sown back in 2013 in Granada, Spain, when a chance encounter with Dr. Meriem El Ghachi at the magnificent Alhambra during a crytallization workshop I was teaching led to things blossoming a few years later. Two years after we had first discussed crystallography approaches to understanding how proteins work, Meriem contacted me asking for help with hers.

Dawn on Charles V palace in Alhambra, Granada, Spain
I suggested that Meriem send us protein, which Dr. Nicole Howe in my lab promptly set up in crystallization trials. The protein was BacA (aka UppP), an enzyme involved in bacterial cell wall synthesis and an antibiotic target. Almost immediately, Nicole got crystals, but to solve the structure of this important enzyme we needed a considerably more focused effort. This led to Meriem spending months working with Nicole in my lab at Trinity College Dublin, where they produced protein and performed crystallization trials on a massive scale.
Unfortunately, the crystals that BacA grew were tiny, challenging to harvest, and did not diffract particularly well following the traditional method of crystal harvesting. This involves poking around in the viscous cubic mesophase in which the crystals grow, picking them out with a harvesting tool and then flash freezing them in liquid nitrogen. We had reasoned that such manhandling could damage the crystals, adversely affecting diffraction quality.
In situ collection. To deal with such issues we developed a method with colleagues at the Swiss Light Source, for what is called in situ data collection. We grow crystals in special plates where diffraction data can be collected directly without ever touching the crystals or the viscous and sticky mesophase in which they grow. We tested this new method with BacA and, like magic, we got beautiful diffraction and a structure to high resolution. This was an important milestone for the in situ method because it was the first structure solved using experimental or de novo phasing methods, as opposed to the less demanding computational phasing approach.
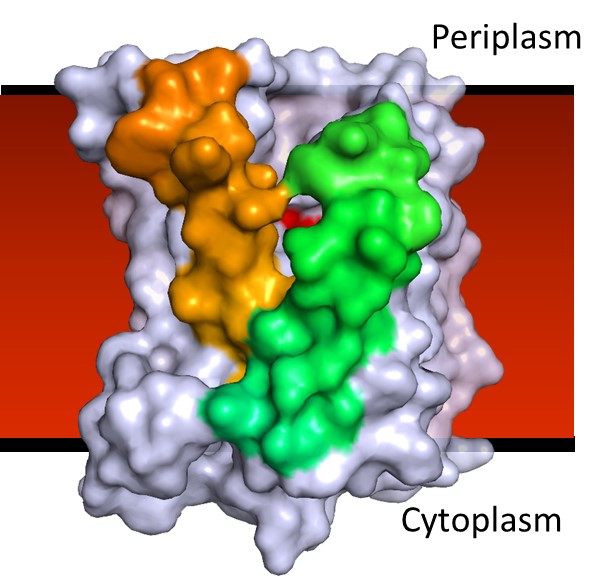
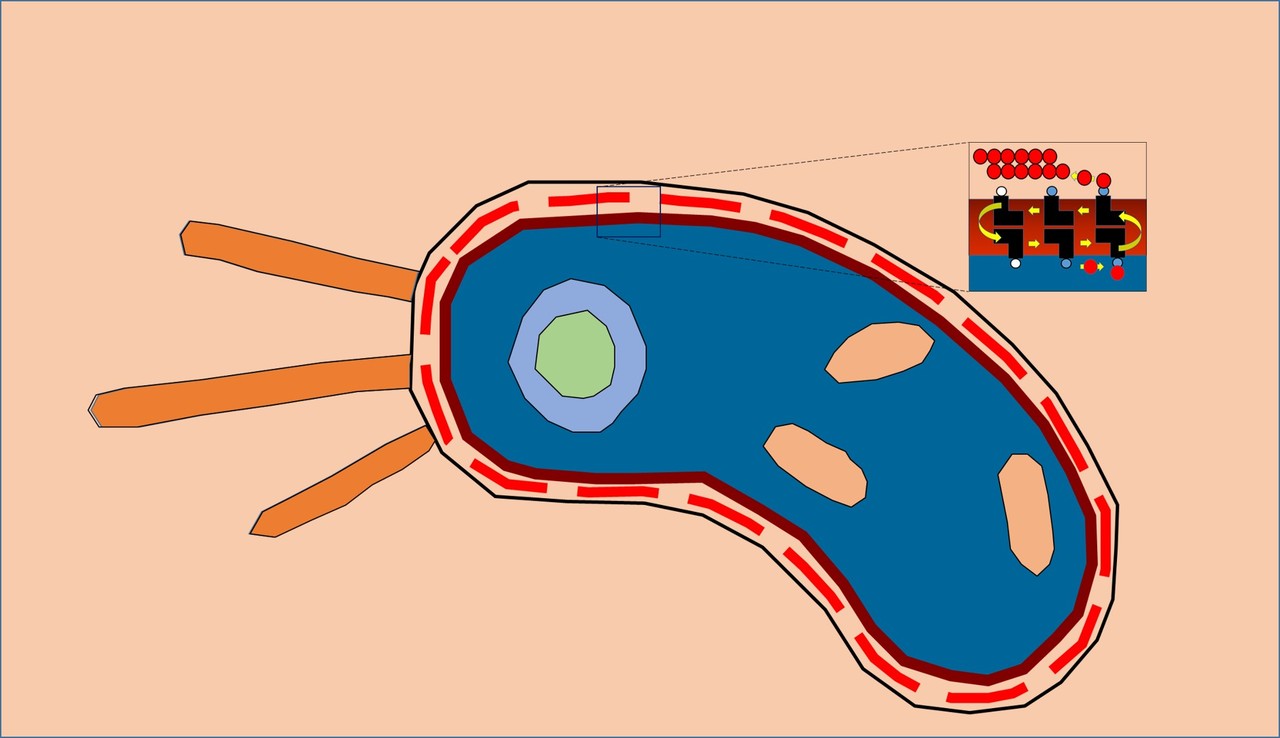
BacA enzyme in the membrane. Active site (red blotch) behind ‘gatekeeping’ helices shown in green and tan. Other helices in the jumble are colored grey.
So now we had our structure of BacA at close to atomic resolution, which meant our structure-function project moved into high gear.
Structures, acting as blueprints of sorts, should provide clues about how proteins work at a molecular level, which can offer insights into how they might be exploited for the design and discovery of therapeutics. In the case of BacA, however, the structure itself presented a fascinating challenge. While the protein is relatively small and consists mostly of helices that partially or fully cross the membrane, those helices were assembled in a confusing jumble. The question then became: How do we make sense of this plumber’s nightmare?
Beguiling symmetry. We identified conserved residues that pinpointed the active site pocket of the enzyme, but lurking in the confusion of helices was a beguiling structural symmetry. It was only as I was out for a stroll along the pier in Dublin Bay, arranging in my head one helix after the other across an imaginary membrane, that the nature of the symmetry jumped out at me: the protein comes in two halves related to one another by a 180˚ axis of rotational symmetry that lies in the plane of the membrane.
Interestingly, the helices from one half of the protein interleave in a non-obvious way with those of the other half. Fortunately, this was not the first time such symmetry had been observed in proteins -- it is not uncommon for transporters and carriers to use internal structural symmetry as part of a rocker-switch mechanism to move cargo, in the form of ions and molecules, across membranes. Luckily for me, Dr. Lucy Forrest at the National Institutes of Health had recently written a most informative review on the topic, which helped make sense of BacA’s symmetry.
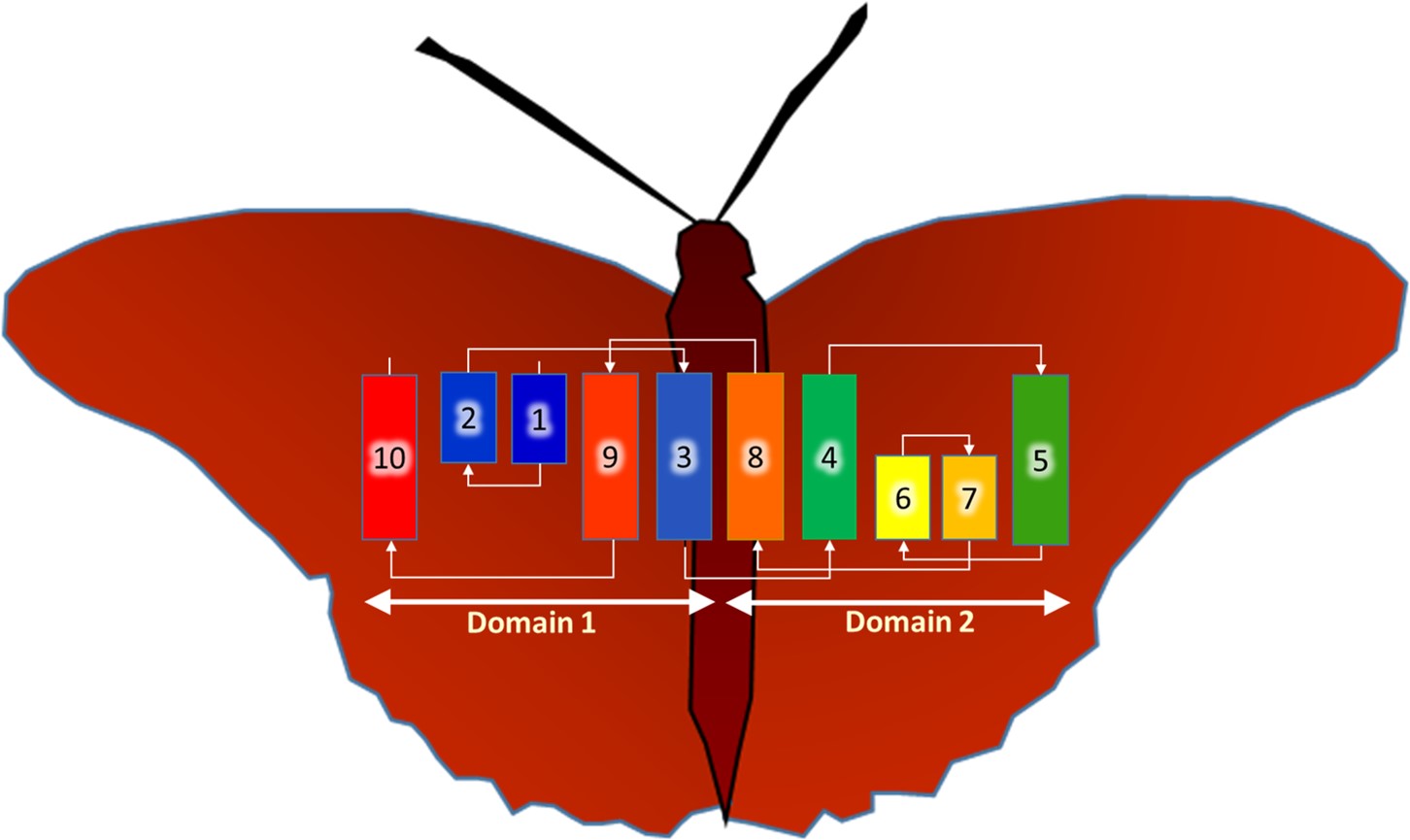
BacA, a jumble of helices -- some long, some short. They twist and turn across the membrane in the order indicated. The final folded form, the functional enzyme, is proposed to emerge when the two, symmetry related domains come together -- the butterfly closes its wings.
Double jobbing. But an additional confounding factor was that BacA is an enzyme; it had no known transporter or channel activities. And so another question arose: Why should an enzyme possess this kind of symmetry if it did not engage in vectoral transmembrane movement?
It was at this point that we began to entertain the possibility that BacA was double-jobbing -- acting as an enzyme and as a flippase where the entity flipped or transported is the product of the reaction it catalyzes. This would make sense because the enzyme reaction takes place facing the extracellular space while the product is needed on -- and must be flipped over to -- the cytoplasmic side of the membrane.
In our paper, we reason back and forth the merits of the double-jobbing hypothesis and how flipping might occur. This includes a ‘swipe card’ proposal where the long lipid molecule shimmies across the membrane with its polar head group slotted between two neighbouring transmembrane helices and its apolar chain extending into the hydrocarbon recesses of the bilayer. However, in the absence of convincing experimental data in support of flippase activity, this remains just a hypothesis.
We are busy now investigating the idea. If it holds up, it would shed light on a flippase activity, long sought-after in the field, and the final player in the peptidoglycan component cycle, which functions by straddling the bacterial plasmamembrane. At the same time, we are hot in pursuit of novel compounds that inactivate BacA to serve as much needed therapeutics to counter the global healthcare threat posed by antibiotic resistance.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Amazing Article