A more sustainable pathway towards urea synthesis with nitrate and carbon dioxide
Published in Sustainability

The world population will increase up to approximately ~10 billion in 2050. This calls for a critical attention due to the population growth which then leads to the potential global food scarcity. Synthetic nitrogen fertilizers are a vital component of intensive agriculture and a necessity for global food production, making them invaluable toward achieving global food security. Of these, urea is the most important nitrogenous fertilizer since available nitrogen is typically the limiting nutrient that inhibits soils from sustaining intensive crop growth. Urea is mainly derived from ammonia (NH3) with carbon dioxide (CO2). This process consumes approximately 80% of NH3. In this regard, the ammonia synthesis from molecular nitrogen and hydrogen through the preeminent Haber–Bosch process plays a key role in feeding humankind, which has been regarded as one of the greatest inventions of the 20th century. But such process still accounts for approximately 2% of global energy consumption and 2% of global CO2 emissions due to the energy-intensive reactions conditions (100–200 bar and 400–500 °C).
To reduce the carbon emissions from ammonia production, it is highly desirable to develop a sustainable approach which can be driven by renewable sources. In recent years, electrocatalytic nitrogen reduction reaction (NRR) has provoked attentions from researchers on directly producing NH3 from atmospheric nitrogen and water at ambient conditions. Despite extensive efforts from our groups and others, challenges still remain in realizing high NH3 yield. Moreover, extra steps are needed to separate and purify the electro-synthesized NH3 in aqueous electrolytes. Further synthesizing urea in industry by the reaction of NH3 with CO2, nonetheless, also relies on the reactions operated under harsh conditions (150–200 °C, 150–250 bar) with extremely large energy consumption. Such disadvantages result in the complexity and impracticality for the subsequent urea synthesis.
So, why not just directly synthesize urea through one-step electrocatalysis approach? To this end, we explored in this work using nitrate (NO3−), a highly desirable nitrogen-containing alternative with lower dissociation energy for the N=O bond (204 kJ mol−1) compared with that for N≡N bond (941 kJ mol−1). Moreover, the reaction we have developed could enable the sustainable synthesis of urea from essentially environmental wastewater (nitrate often found in wastewaters), using renewable electricity at room temperature and ambient conditions.
In our recent work published in Nature Sustainability, we realized the high-selectivity urea production by coupling NO3− (nitrate) with CO2 under ambient conditions (Fig. 1). We developed a novel nanocatalyst indium hydroxide (In(OH)3) as an electrocatalyst for urea production. The catalyst shows facet-dependent activity, and only the {100} facets favour the direct C–N coupling for urea production. We found this special catalyst offer a unique C–N coupling mechanism to offer high energy efficiency and high urea selectivity leading to high yield of urea synthesis.
This work not only develops a sustainable urea production method using nitrate, carbon dioxide, water and sustainable electricity to help address environmental concerns, but also provides deep insights into the fundamental mechanisms for C–N coupling. We believe that the sustainable synthesis of indispensable chemicals will attract much more attentions in the future.
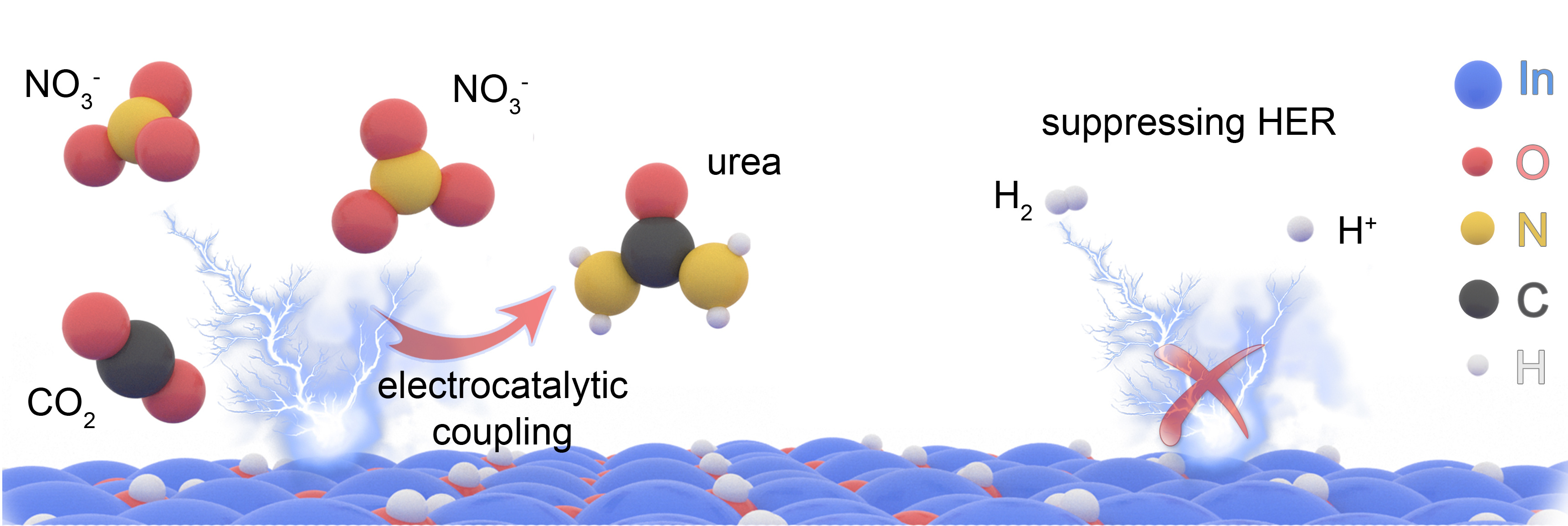
Fig.1 Schematic illustration of electrocatalytic urea production on In(OH)3.
For more details of this work, please see our recent publication in Nature Sustainability:
Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide, https://www.nature.com/articles/s41893-021-00741-3
Follow the Topic
-
Nature Sustainability

This journal publishes significant original research from a broad range of natural, social and engineering fields about sustainability, its policy dimensions and possible solutions.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcement



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in