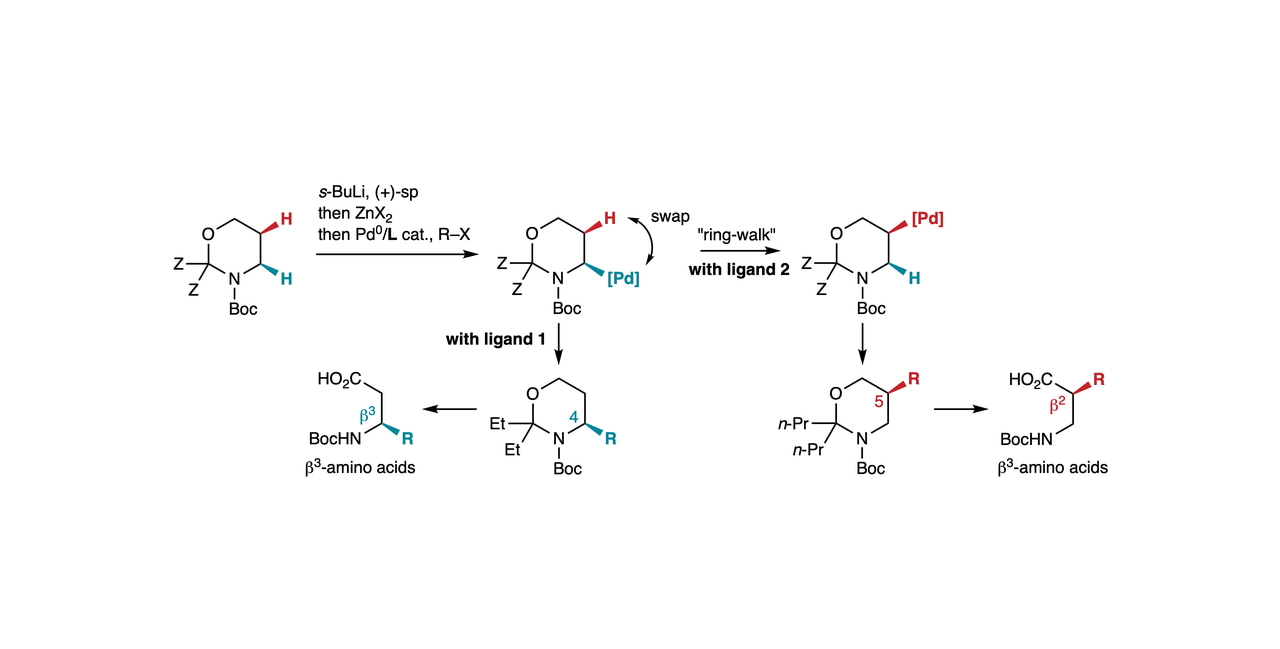
From 2010,[1] our group initiated a program directed toward the functionalization of remote C(sp3)–H bonds via Pd0 catalysis and the so-called “chain-walk” mechanism,[2] which is complementary to C–H activation-based methods.[3] In particular, we reported the implementation of this strategy on cyclic amines (Fig. 1).[4] An appropriate ligand design enabled the regiodivergent arylation of alpha-zincated Boc-piperidines, generated by Boc-directed lithiation and Li-Zn transmetallation, at the C2- and C3-positions.
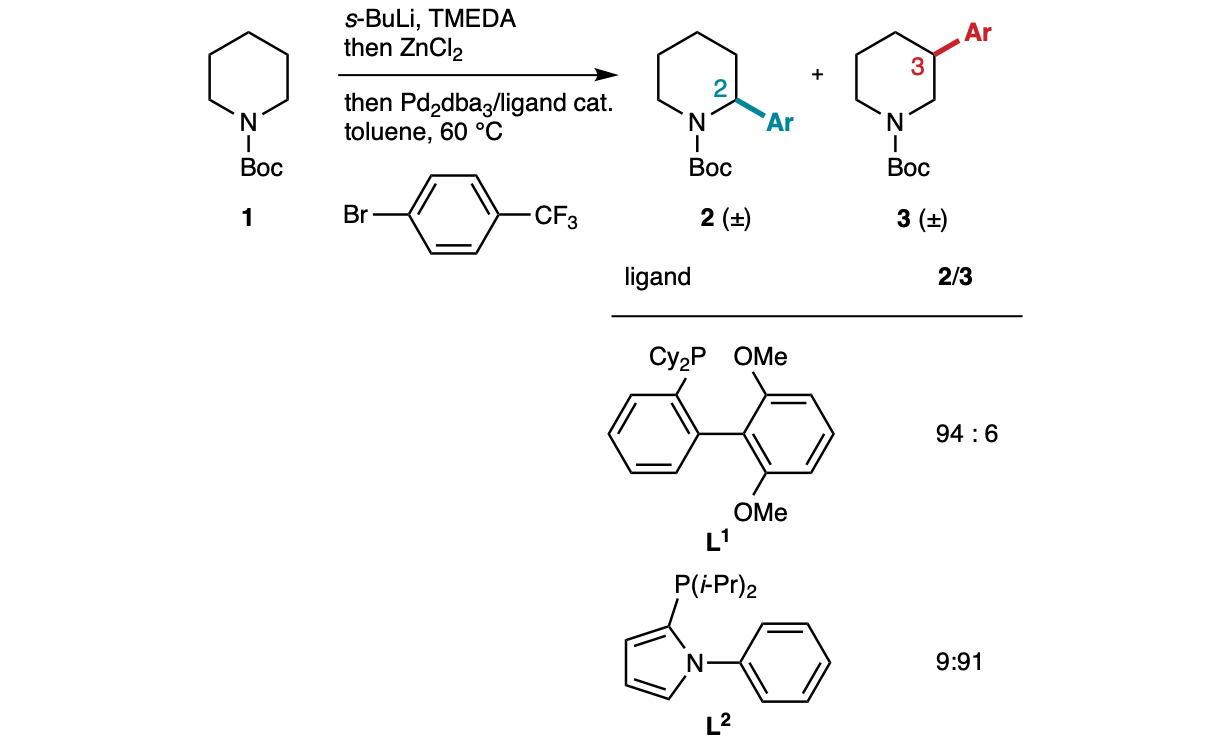
Fig. 1 Ligand-controlled arylation of Boc-piperidine at the C2 and C3-positions.
However, the corresponding products were racemic, and hence a next logically step was to try to design an enantioselective variant. Achieving this requires performing the initial directed lithiation enantioselectively, knowing that all subsequent steps should be stereospecific. Unfortunately, previous work by the Beak[5] and O’Brien[6] groups showed that the enantioselective lithiation of Boc-piperidine using chiral diamines occurs with low yield and moderate enantioselectivity.
Investigations of other cyclic Boc-protected amines led Ke-Feng Zhang and Weilong Lin to discover that Boc-1,3-oxazinanes are lithiated with excellent yield and enantioselectivity using the s-BuLi/sparteine complex. Then, transmetallation to zinc and Negishi coupling in the presence of the bulky phosphine ligand L3, designed to inhibit Pd migration,[7] led to a perfectly C4-selective and highly enantioselective arylation (Fig. 2). Using the smaller ligand L2 already employed in the C3-selective arylation of Boc-piperidine completely switched the selectivity to the C5-position, leading to a range of C5-arylated and alkenylated products with equally good enantioselectivity. Such levels of selectivity were beyond our expectations and probably arise from the particular conformational features of the Boc-oxazinane substrates, which remain to be investigated. The analysis of the absolute configurations of various products confirmed that, after the enantioselective lithiation step, all subsequent steps occur with stereoretention. Mechanistically, the reaction proceeds through organopalladium intermediate A, which “ring-walks” stereospecifically to the next carbon (B) by swapping positions with a cis-located hydrogen atom.
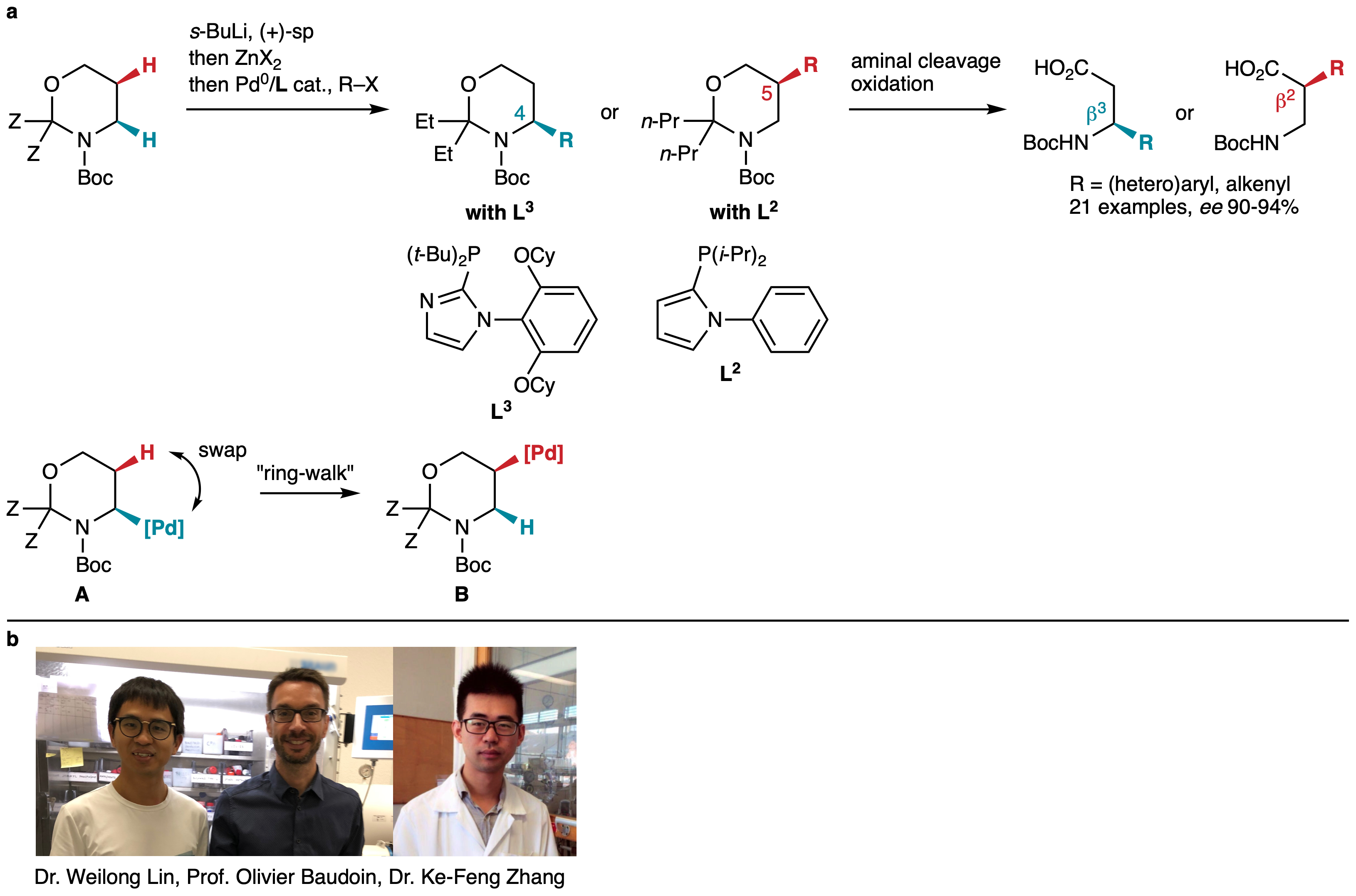
Fig. 2 Ligand-controlled arylation of Boc-1,3-oxazinanes at the C4 and C5-positions and application to the synthesis of beta-amino acids. a Summary of results. b Team members.
When we initially considered Boc-1,3-oxazinanes, we also had in mind that our method would ultimately provide an easy access to chiral beta-amino acids. These are valuable building blocks for the design of new peptidomimetics, which is an important focus of current pharmaceutical research.[8] Indeed, the C4- and C5-functionalized reaction products were easily transformed into enantioenriched beta3- and beta2-amino acids, respectively, upon aminal cleavage and oxidation.
You can read more about this work here: https://www.nature.com/articles/s41929-019-0336-1
[1] Renaudat, A., Jean-Gérard, L., Jazzar, R., Kefalidis, C. E., Clot, E., Baudoin, O. The palladium-catalyzed barylation of carboxylic esters. Angew. Chem. Int. Ed.49, 7261-7265 (2010).
[2] Sommer, H., Juliá-Hernańdez, F., Martin, R., Marek, I. Walking metals for remote functionalization. ACS Cent. Sci.4, 153-165 (2018).
[3] He, J., Wasa, M., Chan, K. S. L., Shao, Q., Yu, J.-Q. Palladium-catalyzed transformations of alkyl C−H bonds. Chem. Rev.117, 8754-8786 (2017).
[4] Millet, A., Larini, P., Clot, E., Baudoin, O. Ligand-controlled b-selective C(sp3)–H arylation of N-Boc-piperidines. Chem. Sci.4, 2241-2247 (2013).
[5] Bailey, W. F., Beak, P., Kerrick, S. T., Ma, S., Wiberg, K. B. An experimental and computational investigation of the enantioselective deprotonation of Boc-piperidine. J. Am. Chem. Soc.124, 1889-1896 (2002).
[6] Stead, D., Carbone, G., O’Brien, P., Campos, K. R., Coldham, I., Sanderson, A. Asymmetric deprotonation of N-Boc piperidine: React IR monitoring and mechanistic aspects. J. Am. Chem. Soc.132, 7260-7261 (2010).
[7] Zhang, K.-F., Christoffel, F., Baudoin, O. Barbier-Negishi coupling of secondary alkyl bromides with triflates and nonaflates. Angew. Chem. Int. Ed. 57, 1982-1986 (2018).
[8] Cabrele, C., Martinek, T. A. , Reiser, O., Berlicki, L. Peptides containing beta-amino acid patterns: challenges and successes in medicinal chemistry. J. Med. Chem. 57, 9718-9739 (2014).
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in