A New Hope Beyond Halide Perovskites?
Silicon has long dominated the solar cell industry, but its efficiency is now approaching its theoretical maximum of 29.4%. To break this ceiling, researchers are exploring new materials to complement silicon in tandem solar cells, which stack layers to capture more of the solar spectrum. However, finding the right materials is no small feat, as defects in their crystalline structure often limit their efficiency. Silicon’s success stems from the microchip industry’s ability to produce ultra-high-quality wafers. Unfortunately, this level of quality isn’t available for newer materials, slowing their development. A prime example is CZTS, a material made from copper, zinc, tin, and sulphur or selenium. CZTS is attractive because it is abundant, non-toxic, and low-cost. However, its high susceptibility to defects has hampered its progress as a practical solar material.
Halide Perovskites: A Breakthrough with Limitations
A more promising alternative has emerged in the form of halide perovskites, whose efficiency has skyrocketed by 579% in just 12 years (2009–2021). For comparison, silicon solar cells saw only a 57% improvement during their peak years (1983–2009).
Halide perovskites are remarkable for their “defect tolerance,” although the exact reasons behind this remain under debate. These materials follow a unique structural formula (ABX3) that gives rise to several fascinating properties. Their “soft” crystalline structure resembles a flexible gel that deforms easily, and when their symmetry breaks, it unlocks four exotic properties:
- Ferroelectricity: Similar to how ferromagnetic materials respond to magnetic fields, ferroelectric materials develop polarized domains when exposed to electric fields. These domains act as pathways for efficient electron extraction, reducing energy loss during the conversion of sunlight to electricity.
- Rashba Effect: This phenomenon occurs when electrons with different spins have slightly different energy levels due to structural asymmetry. This effect makes it harder for electrons to recombine prematurely, effectively extending their lifespan. The longer lifetime allows more time for electrons to be captured and used in generating electricity.
- Large Polarons: These are quasi-particles that form when electrons interact with vibrations in the crystal lattice (known as phonons). Large polarons create a protective “cloud” around charge carriers, allowing them to move more efficiently through the material with less disruption from defects.
- Hot Phonon Bottleneck: In simpler terms, this effect slows down the loss of energy from excited electrons. As a result, more of the absorbed sunlight can be converted into electricity rather than being lost as heat.
The Downsides of Perovskites
While the perovskite structure offers exciting possibilities, not all perovskites are suitable for solar cells. For example, most oxide perovskites (such as those based on titanium or zirconium oxides) have a wide band gap, meaning they cannot absorb enough of the solar spectrum to be efficient for photovoltaic applications. In contrast, halide perovskites have ideal band gap energies for sunlight absorption, making them more effective in solar cells.
Despite their advantages, halide perovskites also have significant drawbacks:
- Toxicity: Most contain lead, which poses environmental and health risks.
- Instability: Halide perovskites degrade under heat, light, and air exposure. Encapsulation can offer limited protection, but their inherent instability makes long-term commercial applications challenging.
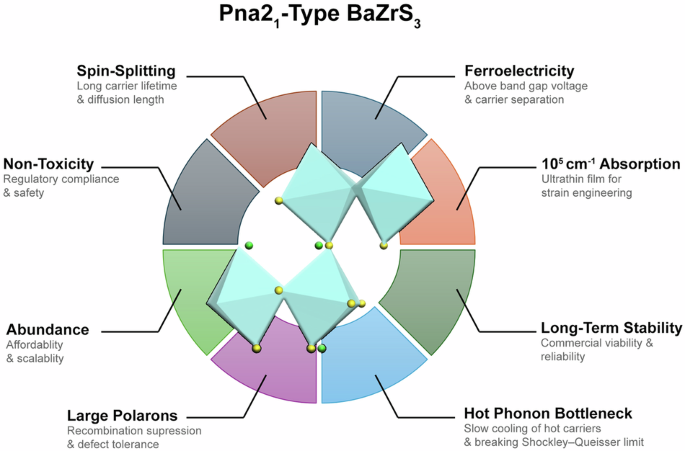
BaZrS3 (BZS): A Promising Alternative
While halide perovskites receive much attention, other types of perovskites—specifically chalcogenide perovskites like BaZrS3 (BZS)—offer promising alternatives. BZS avoids the issues of toxicity and instability while retaining favourable properties for solar applications. It was first predicted as a potential solar material 15 years ago, but no functional solar cells using BZS have been reported yet.
Recent research published in Communications Materials by Yaghoubi et al. from the Australian Centre for Advanced Photovoltaics (ACAP), has reignited interest in BZS. Using state-of-the-art atomic simulations performed on Gadi, one of the supercomputers at Australia’s National Computational Infrastructure (NCI), the study found a weakly ferroelectric Pna21 phase. By applying strain to the material, researchers showed that its properties could be enhanced to exhibit the same exotic traits as halide perovskites, such as ferroelectricity, Rashba splitting, and large polaron formation.
The newly discovered perovskite shows weak ferroelectricity, but when strained, it gains exotic properties that can significantly enhance solar cell performance.
The Path Forward for BZS
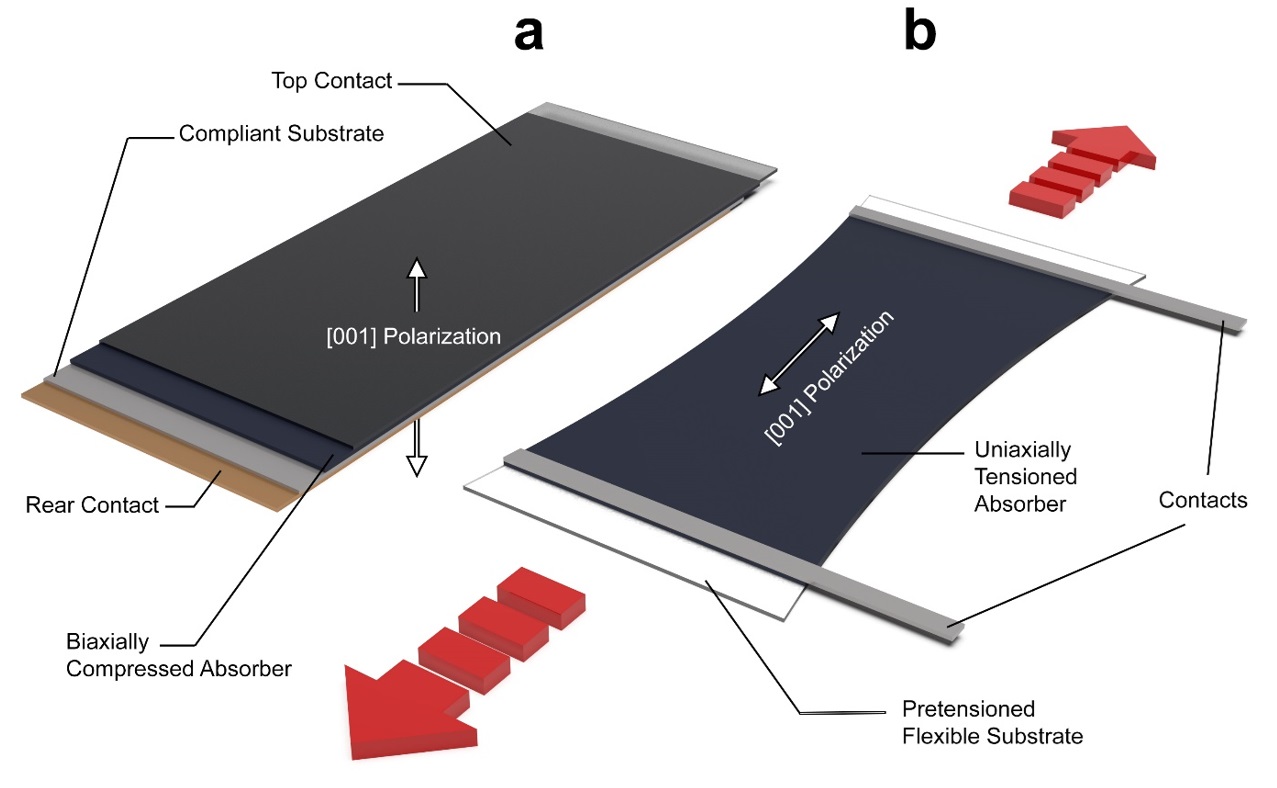
The authors propose a bold idea: stacking up to 100 ultrathin, semi-transparent layers of strained BZS to maximize its energy efficiency. When paired with existing commercial solar cells, this could achieve efficiencies exceeding 38%, far surpassing current silicon-based technologies.
Despite its potential, BZS faces significant challenges:
- Difficult Production: Its high stability, while advantageous for durability, makes it harder to produce as a thin film.
- Oxygen Sensitivity: Barium and zirconium, the key components of BZS, have a strong affinity for oxygen. This makes the high-temperature production process highly sensitive to contamination, requiring precise control.
Looking Ahead
Pna21 BaZrS3 represents a new frontier in solar technology, combining stability, non-toxicity, and potentially high efficiency. While halide perovskites have demonstrated the potential of perovskite materials, their commercial viability is hindered by toxicity and stability concerns. BZS could overcome these limitations, paving the way for the next generation of sustainable and efficient solar cells. However, much work remains to refine its production processes and unlock its full potential for widespread use.
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Fundamental science and applications of silk proteins
Publishing Model: Open Access
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in