In 2020, our discovery that methylmalonic acid (MMA) was an oncometabolite linking old age to poor cancer outcomes was followed by much excitement (mostly on our part) as well as buzz in the press, which described our identified culprit as “an acid produced during digestion”(1). While not technically untrue, I found this description a bit reductive and slightly unsettling, particularly because we did not, in fact, know why MMA was increased in the blood of older people. That question remains to be answered, but meanwhile, we were reminded that it is often endogenous biological processes that are hijacked by cancer cells to support their growth and deadly spread. Take epithelial-to-mesenchymal transition (EMT), for instance, a developmental program that occurs during the formation of the embryo, that cancer cells commandeer to gain metastatic abilities. We considered the potent effect of this metabolite, MMA, in driving cancer aggressiveness, and wondered if cancer cells may hijack the endogenous “digestive,” or more accurately, the metabolic process that naturally produces it, to benefit their own progression. As we would soon discover, this was in fact the case, and so the seeds for this paper began to sprout.
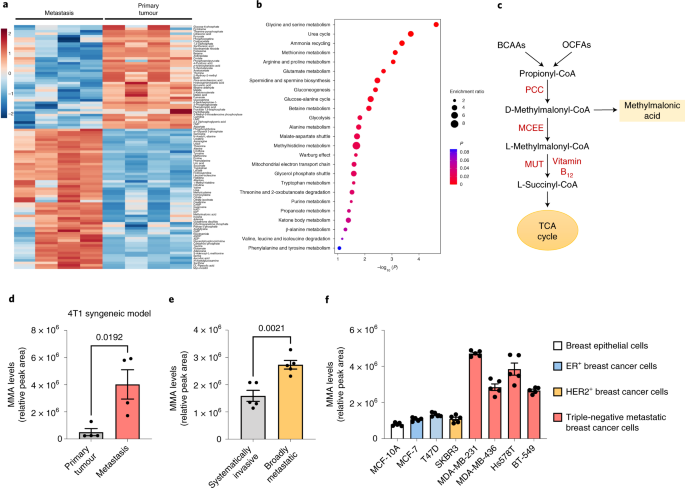
Methylmalonic acid is produced as a byproduct of propionate metabolism, an excess metabolite that accumulates during the cellular conversion of certain amino and fatty acids into energy. It can be used as an index of vitamin B deficiency, and is overproduced in a family of extremely rare congenital disorders called methylmalonic acidemias. Beyond these applications, however, no one paid much attention to it at all, and it remained largely in obscurity, certainly in the cancer field, until our Nature 2020 paper brought it into the spotlight. Our new study demonstrates for the first time that MMA is not only increased in the blood of the elderly by some passive, unknown mechanism, and giving cancer cells a boost as it serendipitously encounters them, but rather it is manufactured and amassed by cancer cells themselves as they advance towards a more aggressive and metastatic state. We compared metastases to primary tumors, aggressive triple negative breast cancer (TNBC) cell lines to less malignant ER+ breast cancer cell lines, cells treated with metastatic inducers and cells that were not, and found that MMA was always increased in the more aggressive cells. By using isotope labels to track the fate of amino acids that we then fed to cells, we confirmed that the excess MMA being produced by aggressive cells was made through the propionate metabolism pathway.
There are many factors known to induce the changes in cancer cells that allow them to metastasize. For example, hypoxia and nutrient deprivation, conditions that cancer cells endure as they become overcrowded and suffocated in their primary niches, are sufficient to drive certain cancer cells to escape and embark for new organs to colonize. We investigated the regulation of propionate metabolism in two model systems for the induction of metastasis, one using overexpression of an ERK mutant shown to drive EMT, and one treating cells with the metastatic inducers TGFb/TNFa. In both systems, we found that methylmalonyl-CoA epimerase (MCEE), the enzyme downstream MMA in the propionate metabolism, was reduced. As it turned out, the absence of MCEE downstream was causing a build-up of MMA upstream in the propionate metabolism pathway, which was subsequently inducing aggressive qualities in cancer cells.
We found that when we manipulated the propionate metabolism pathway to produce more MMA, whether by increasing expression of enzymes upstream of MMA, knocking down expression of enzymes downstream of MMA, or blocking the ability of downstream enzymes to function (such as by depletion of vitamin B12), cancer cells acquired aggressive markers, increased invasive and migratory abilities, and increased metastatic potential in vivo. Interestingly, while MCEE was the common point of regulation in the early metastasis model systems we utilized, we have since found increasing evidence to suggest that propionate metabolism is dysregulated on many multiple different levels by various metastatic inducers to increase MMA production and accumulation. Additionally, equipped with the knowledge that MMA is specifically and locally produced by tumors, we are also investigating the effects of MMA in the tumor microenvironment. Without providing too many spoilers, our study demonstrates for the first time the previously unknown role of an endogenous metabolic pathway, propionate metabolism, in cancer progression, and paves new avenues for research that we have already begun to explore.
- A. P. Gomes et al., Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature 585, 283-287 (2020).
Follow the Topic
-
Nature Metabolism

This journal publishes work from across all fields of metabolism research that significantly advances our understanding of metabolic and homeostatic processes in a cellular or broader physiological context, from fundamental cell biology to basic biomedical and translational research.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in