A promising nanotechnology to unlocking bladder cancer treatment
Published in Biomedical Research
This work began with a conversation between Dr. Wanwan Li with Dr. Guoliang Yang, a clinician who specializes in the treatment of bladder cancer, as Dr. Wanwan Li’s group from Shanghai Jiao Tong University, has long been expecting to promote the technique of X-ray-activated photodynamic therapy toward clinical translations. After this inspiring conversation and brainstorm, we realized that the interdisciplinary and complementary background of both groups would promote the nanotechnology from laboratory to the clinic instead of just concept demonstration.
X-ray-activated photodynamic therapy is clinically promising
X-rays have emerged as an ideal clinical excitation source with “unlimited” penetration capacity and was reported in 2006 with promises to promote the clinically approved photodynamic therapy (PDT) towards deep-seated tumours1. This technique has been termed X-ray-activated PDT (X-PDT) with a nanoscintillator-photosensitizer structure. During the past several years, we have established several other efficient nanoplatforms for X-PDT 2,3. However, previous work in this field mainly focused on developing new nanomaterials and demonstrating X-PDT concept on subcutaneous xenograft tumour mouse models, which was unfordable for clinical use. Thus, for us, validating the efficiency of X-PDT in the treatment of autochthonous bladder cancer is both challenging, attracting, and meaningful in advancing the clinical translation of X-PDT.
Non-invasive ladder cancer treatment is to be settled
Bladder cancer is a highly prevalent malignancy in elderly individuals (median age of 73)4. Conventional approaches for non-muscle invasive bladder cancer (NMIBC) that occupy more than 75% include surgical resection, cystoscopy-assisted photomedicine, and high-dose radiation5. However, NMIBC patients are still at high risk of recurrence (73% in 5 years). Consequently, the diagnosis, treatment, and five-year survival rates have not been largely improved since the 1990s. Therefore, developing non-invasive and full-course imaging-navigated efficient therapeutic approaches are urgently appealing to combat NMIBC (Figure 1). Notably, nanomaterials can be administered through intravesical instillation to avoid the long-debated toxicity associated with systemic administration.
Figure 1 | Schematic cartoon illustrates the proposed nanotechnology against NMIBC.
Imaging-navigated X-PDT of bladder cancer is highly efficient
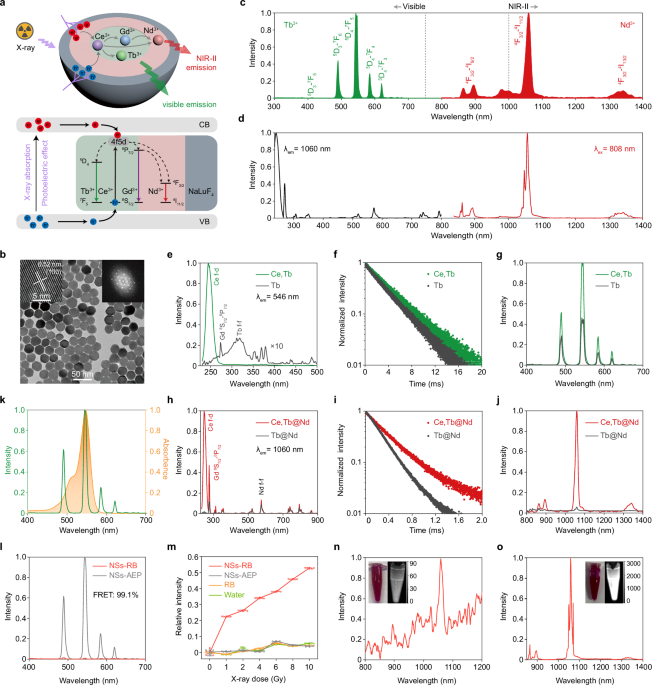
Building upon the concept, we designed nanotransducers with a new dual-mediation role of Ce3+ to achieve the first concurrent visible and the second near-infrared (NIR-II) radioluminescence (Figure 2a). Nanotransducers were engineered by introducing Tb3+ and Nd3+ as the dual activators with an inert outer layer of NaLuF4 to enhance the photoelectric effect upon X-rays. X-ray energy absorption, distribution, and conversion processes in nanoscintillators were studied to boost both radioluminescence (Figure 2b-d).
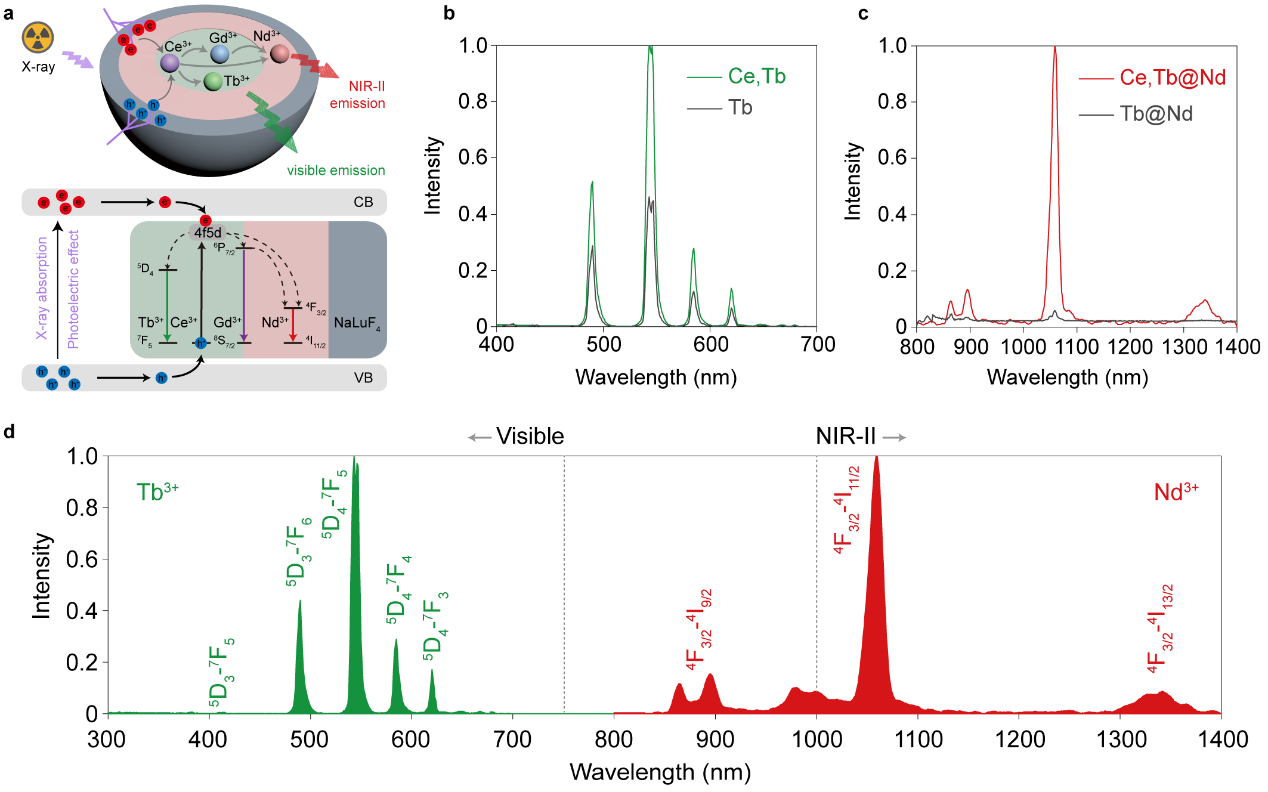
Figure 2 | Illustration of luminescence mechanism and X-ray-activated green and NIR-II emissions of lanthanide-doped nanoscintillators.
To mimic a fit-for-purpose technique in the clinic, we selected female C57BL/6 mice bearing autochthonous bladder tumours that have highly analogous differential molecular subtypes to human bladder cancer (Figure 3a,b)5. Coincidentally, Dr. Guoliang Yang’s group is skilled in this mouse model. We also conjugated the nanotransducers with the tumour-homing cyclic arginine-glycine-aspartate peptide (cRGD) to improve specific targeting capability and tumour accumulation, as demonstrated by ex vivo NIR-II fluorescence imaging of tumour tissues and frozen slices (Figure 3c). Upon single X-ray irradiation, green radioluminescence of nanotransducers can promote robust PDT with restored immune homeostasis, eliminate tumours, and inhibit recurrence (Figure 3d), whereas concurrent NIR-II fluorescence enables real-time imaging for in situ post-operative tumour evaluation.
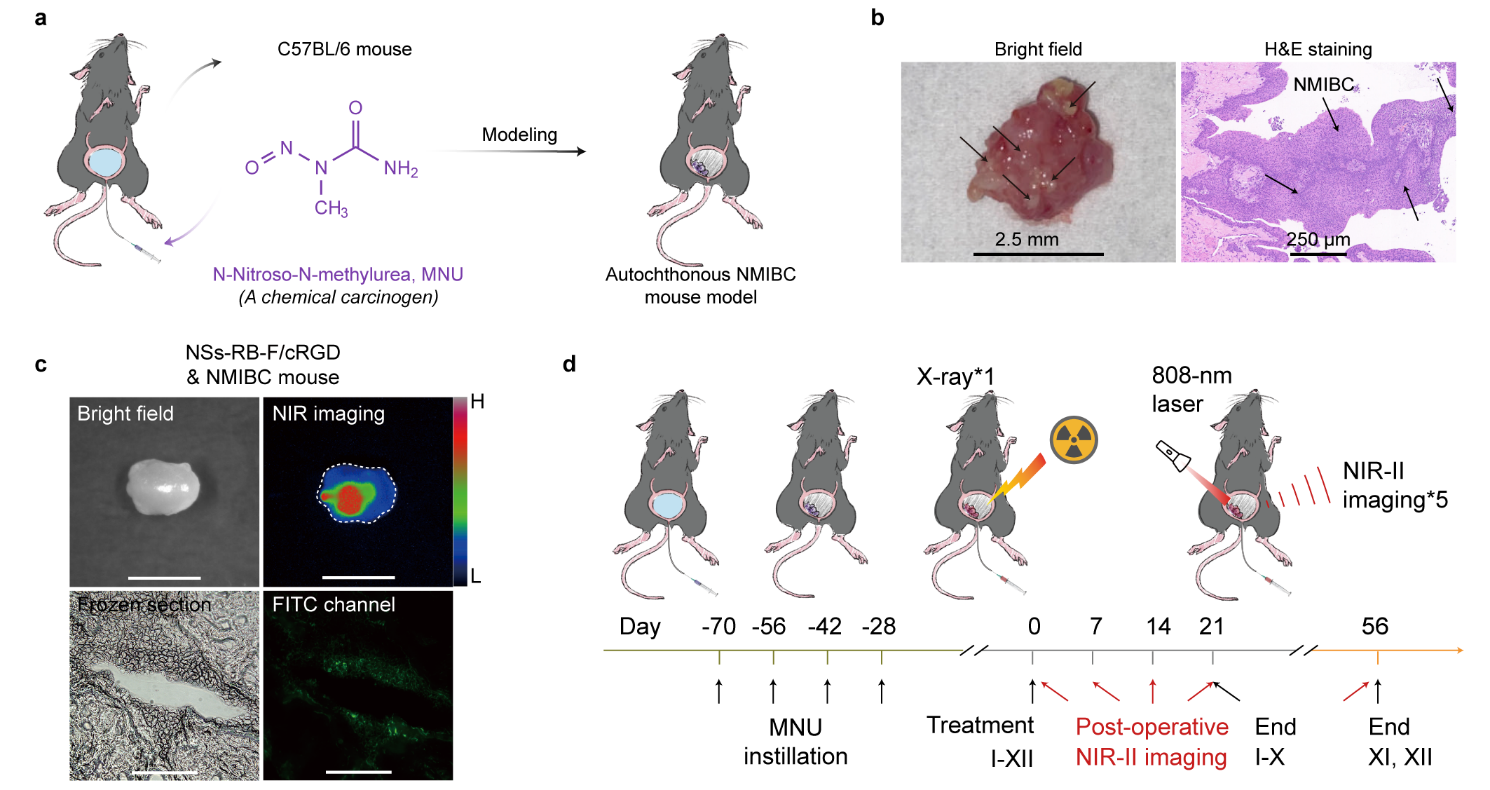
Figure 3 | Establishment of autochthonous bladder tumour mouse model, specific targeting capability, and single-fractionated PDT with nanotransducers.
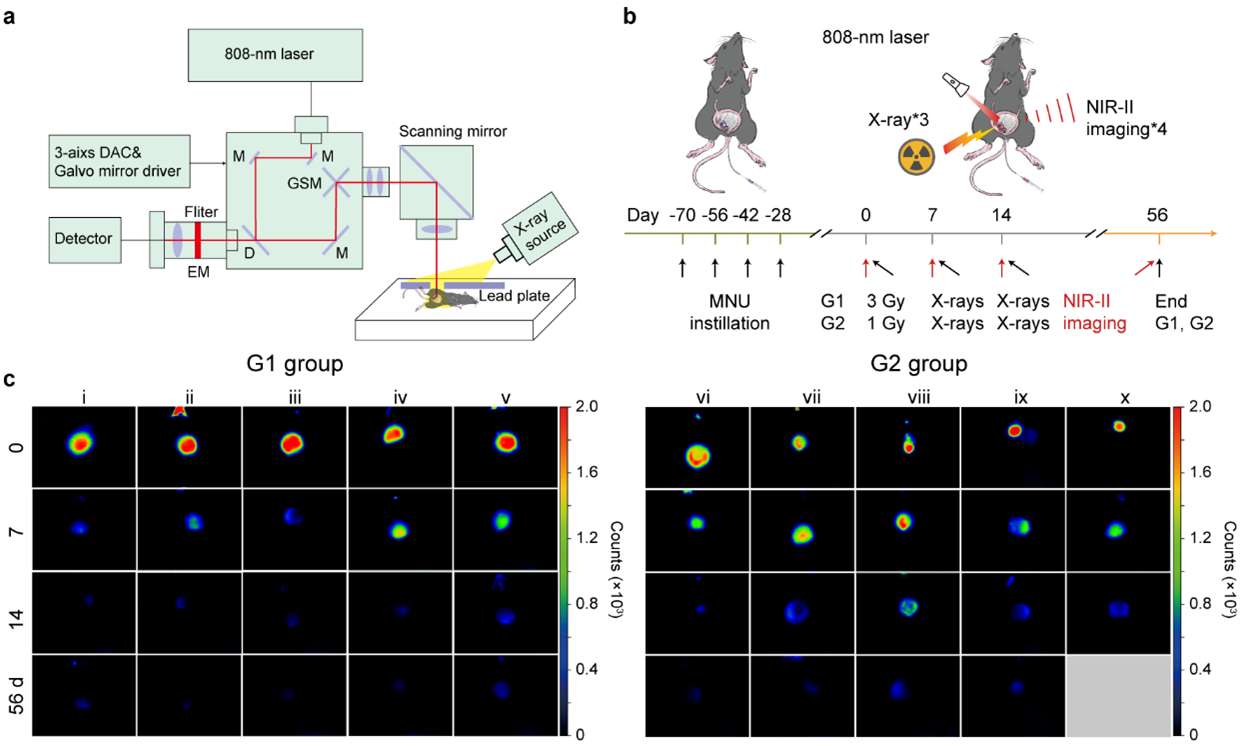
In summary, this work proved the success of NIR-II imaging-navigated f-PDT of clinically alike autochthonous bladder tumours with efficient tumour regression, inhibited recurrence, restored immune homeostasis, and prolonged survival. We believe that this non-invasive full-course imaging-navigated on-demand therapeutic strategy has great clinical value in revolutionizing bladder cancer treatment paradigms.
References:
1. Chen, W. & Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 6, 1159–1166 (2006).
2. Yu, X. et al. CT/MRI-guided synergistic radiotherapy and X-ray inducible photodynamic therapy using Tb-doped Gd-W-nanoscintillators. Angew. Chem. Int. Ed. 58, 2017–2022 (2019).
3. Jiang, Z. et al. Antiangiogenesis combined with inhibition of the hypoxia pathway facilitates low-dose, X‑ray-induced photodynamic therapy. ACS Nano 15, 11112–11125 (2021).
4. Hoogstraten, L. M. C. van et al. Global trends in the epidemiology of bladder cancer: challenges for public health and clinical practice. Nat. Rev. Clin. Oncol. 20, 287–304 (2023).
5. Bosch, S. van den & Witjes, J. A. Long-term cancer-specific survival in patients with high-risk, non–muscle-invasive bladder cancer and tumour progression: a systematic review. Eur. Urol. 60, 493–500 (2011).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in