A PurFect strategy for tuberculosis drug discovery?
Published in Microbiology, Cell & Molecular Biology, and Pharmacy & Pharmacology
Tuberculosis (TB) continues to be one of the world’s most serious infectious diseases, causing around 10 million infections and over 1.25 million deaths in 2023 alone. Despite improvements in treatment, the disease remains difficult to control, especially as drug-resistant forms of TB are becoming more common. Many current treatment regimens involve taking multiple antibiotics over many months, often with side effects that make it hard for patients to stick with the full course. This not only affects recovery but also contributes to the spread of drug-resistant TB. There is a pressing need for new, more effective drugs that work in different ways from existing treatments.
Our study introduces a new potential TB drug strategy exemplified by JNJ-6640, which kills Mycobacterium tuberculosis, the bacteria that causes TB in a way that has never been done before. It works by blocking a bacterial enzyme called PurF, which plays a critical role in the production of molecules called purines. Purines are essential building blocks for DNA and RNA, the genetic material of cells, as well as for energy storage and cell signalling. All living cells need purines to grow and survive, and bacteria like Mycobacterium tuberculosis are no exception. Bacteria make purines using a specific metabolic route known as the de novo purine biosynthesis pathway. If PurF, the first enzyme in the pathway, is blocked, the bacterium can’t make purines from scratch, which severely hampers its ability to survive. This made PurF an intriguing but previously unexplored target for drug development.
In our study, we discovered the molecule, JNJ-6640, through a method known as whole-cell screening, where thousands of chemical compounds are tested against live bacteria to see which ones can stop them from replicating. Once JNJ-6640 showed strong anti-TB activity in lab tests, we used a range of advanced techniques, including genetic analysis, protein studies and microscopy, to confirm that it specifically targets and blocks PurF in Mycobacterium tuberculosis.
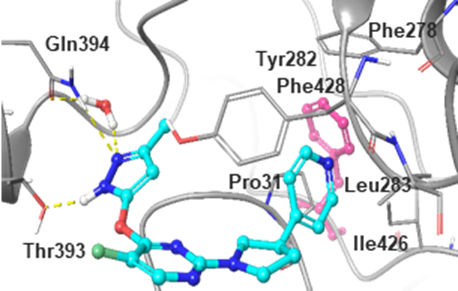
Computational model showing a prediction of how JNJ-6640 may bind to PurF (Fig. 2b)
One major concern with targeting PurF is that TB bacteria might "rescue" themselves by taking purines, or the building blocks to make them, from their surroundings using what's called the purine salvage pathway. If purines from the host (i.e. the patient) could make up for the block in purine synthesis, the drug would be less effective. To address this, we measured purine levels in lung tissue from both humans and mice, the most relevant site for TB infection, and showed that these levels were too low to compensate for the loss of PurF function. In other words, TB bacteria can't rely on salvaging purines in the body to survive if this pathway is blocked.
Importantly, JNJ-6640 wasn’t just effective in lab cultures. We tested it in mouse models of TB using a long-acting injectable form of the drug and showed that it significantly reduced the number of TB bacteria in the lungs. These results demonstrate that blocking PurF is also a viable way to kill TB bacteria in living organisms. The findings also suggest that adding a PurF-targeting molecule like JNJ-6640 to existing treatment regimens could make them more effective, especially against drug-resistant strains of TB.
In summary, our work highlights a promising new strategy in TB drug development. By targeting a previously untapped metabolic pathway, we have not only found a new way to kill TB bacteria but also provided strong evidence that this approach could help transform how we treat this deadly disease, particularly in its most difficult and drug-resistant forms.
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in