A randomised controlled non-inferiority trial to compare the efficacy of ‘HPV screen, triage and treat’ with ‘HPV screen and treat’ approach for cervical cancer prevention among women living with HIV
Published in Cancer and Biomedical Research

In 2013, the World Health Organisation (WHO) recommended use of an HPV test for screening for cervical cancer. The WHO suggested 2 algorithms when screening is done with an HPV test; ‘HPV screen and treat’ when all HPV positive women are treated and ‘HPV screen, triage and treat’ when HPV positive women undergo a triage test and are treated when the triage test is also positive. The currently available tests for triage are visual inspection of the cervix with 5% acetic acid (VIA), cytology, colposcopy or partial genotyping. The most feasible triage test in the low- and middle-income countries is VIA.
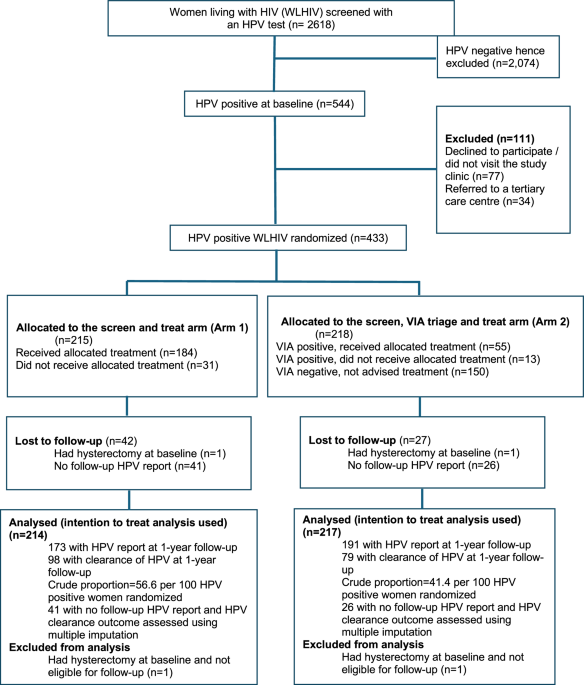
We have reported the results of a randomized controlled trial to compare ‘HPV screen and treat’ (Arm 1) and ‘HPV screen, triage and treat’ (Arm 2) strategies in women living with HIV (WLHIV), using VIA as the triage test. Treatment was offered to all HPV-positive women in Arm 1 and to VIA-positive women in Arm 2 with either thermal ablation or large loop excision of transformation zone (LLETZ). All women underwent a repeat HPV test one year after randomization. The primary outcome was non-inferiority of HPV clearance of Arm 2 at one-year follow up when compared to Arm 1. At follow-up, HPV-positive women in either arm were evaluated with colposcopy and biopsies with detection of CIN 2 or worse (CIN 2+) lesions as the secondary outcome to compare the two algorithms.
At baseline, CIN 2/3 lesions were detected in 16.7% and 13.3% women in Arm 1 and Arm 2 respectively. HPV clearance was observed in 56.6% (95%CI 48.9-64.1) women in Arm 1 and 41.4% (95%CI 34.3-48.7) women in Arm 2 at follow-up in the intention-to-treat population (P=0.004). ‘HPV screen, VIA triage and treat’ strategy was non-inferior to the ‘screen and treat’ strategy as the lower bound of the 95% confidence interval from the regression model was greater than 0.49 in both intention-to-treat analysis (RR 0.73, 95%CI 0.59-0.91) and per-protocol analysis (RR 0.74, 95%CI 0.60-0.93) according to the pre-specified analysis plan. Although non-significant, there was 58% and 48% increased risk of CIN 2+ disease in the ‘screen, triage and treat arm’ when compared to the ‘screen and treat’ arm in the ‘intention-to-treat’ and ‘per-protocol’ analysis respectively.
The WHO 2021 guidelines suggested using HPV screen, triage and treat algorithm in WLHIV using a modelling exercise and our study has addressed an important knowledge gap. We have shown that 'HPV screen, triage and treat' is non-inferior to the 'HPV screen and treat'. At the same time we have demonstrated the advantages of the 'HPV screen and treat' algorithm in WLHIV and it is feasible in countries where the health system can manage the referrals of HPV positive women.
The study was initiated when the first wave of Covid-19 pandemic started declining in western India. The study was continued throughout the 2nd and 3rd wave of Covid-19 pandemic when screening was performed in a mobile screening unit outside the ART centres and then in a makeshift clinic where cervix biopsies and ablative treatment were managed because the hospital was overflown with Covid-19 patients. This demonstrates the feasibility of implementing the ‘screen and treat’ strategy in the mobile screening unit as well as in a makeshift clinic.
Cervical cancer screening particularly with an HPV test and appropriate management is a life saving intervention for WLHIV and must be a standard of care for them at all the antiretroviral centres in India and other low- and middle-income countries.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in