A smart insulin-sensor device for correcting insulin resistance
Published in Bioengineering & Biotechnology

During my PhD and postdoc time in Prof. Dr. Martin Fussenegger’s group, my research interest focused on the engineering of synthetic prosthetic gene networks for the treatment of metabolic disorders. As a result of extensive animal experiments, I observed that the blood-insulin levels in type-2 diabetic mice were much higher than those in wild-type mice. After consulting the literature, I became aware of the fact that there exists a metabolic disorder called insulin resistance. It is a medical condition in which cells fail to respond properly to insulin and the body needs to produce more insulin to keep relative glucose homeostasis. As a consequence, blood-insulin levels remain extremely high [1-4]. This triggered my curiosity and ambition to engineer a smart insulin-sensor circuit that can sense, monitor and profile insulin levels in the blood stream while coordinating insulin-inducible production of adiponectin, which has been reported to reverse the insulin-resistance syndrome (Figure 1).
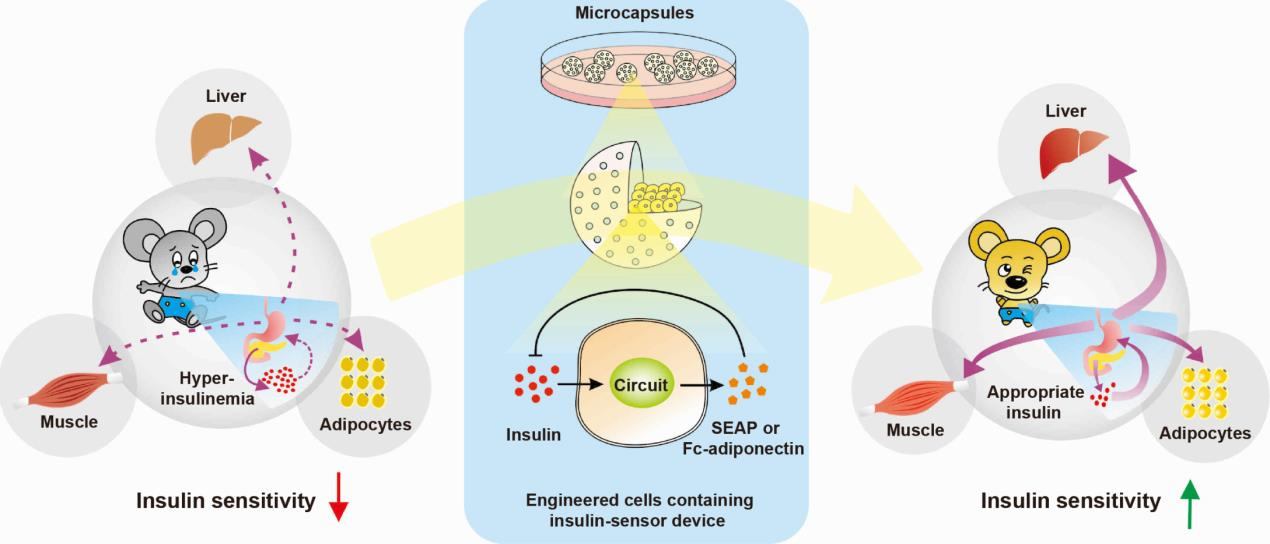
Figure 1. Schematic of the strategy to treat insulin resistance. Microencapsulated engineered cells containing the designer insulin-sensor circuit implanted into insulin-resistant mice quantify blood-insulin levels, detect hyperinsulinaemia and trigger dose-dependent adiponectin production, which restores insulin sensitivity and attenuates the insulin-resistance syndrome.
In order to test this idea, we chose a synthetic biology-inspired approach to design and create an insulin-sensor device, which we validated both in vitro and in vivo. Our in vitro experimental data confirmed that the insulin-triggered transgene expression levels could be fine-tuned and reliably switched ON and OFF by alternating the presence or absence of insulin. We then validated the diagnostic capacity of our synthetic insulin-sensor circuit using serum with high insulin levels from insulin-resistant mice and obesity-induced insulin-resistant patients. To confirm the insulin-triggered transgene expression in vivo, microencapsulated engineered HEK-293 cells containing the insulin-sensor device were intraperitoneally implanted into three different insulin-resistant mouse models (ob/ob, db/db and DIO). Our data confirmed that the synthetic insulin-sensor circuit could indeed sense high insulin concentrations in these three animal models of insulin resistance.
In order to evaluate the long-term therapeutic efficacy of the synthetic insulin-sensor device, we have produced the cell line HEKIR-Adipo, which is stably transgenic for the insulin-sensor circuit. The microencapsulated HEKIR-Adipo cells were intraperitoneally implanted into insulin-resistant ob/ob and DIO mice, where they self-sufficiently detected high insulin levels and produced, secreted, and systemically delivered adiponectin, which substantially decreased blood-insulin levels, serum-lipid levels, insulin-resistant index, food intake and body weight [5].
In short, we created an insulin-sensor-effector device that is able to correct insulin resistance and associated metabolic dysfunctions in different mouse models. This designer circuit has all it takes to develop into a therapy for early stages of diabetes. Self-sufficient therapeutic gene circuits that coordinate disease-marker monitoring with the production of protein pharmaceuticals may represent a new era in modern personalized medicine.

Figure 2. Three of the authors involved in the research to treat experimental insulin resistance. From left to right: Prof. Dr. Haifeng Ye, Mr. Mingqi Xie, and Prof. Dr. Martin Fussenegger.
Our paper (also ref. 5 below): Ye, H. et al. Self-adjusting synthetic gene circuit for correcting insulin resistance. Nat. Biomed. Eng. 1, 0005 (2016).
References:
- Johnson, A. M. & Olefsky, J. M. The origins and drivers of insulin resistance. Cell 152, 673-684 (2013).
- Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852-871(2012).
- Weyer, C. et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86, 1930-1935 (2001)
- Gao, H. et al. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes 62, 1338-1344 (2013)
- Ye, H. et al. Self-adjusting synthetic gene circuit for correcting insulin resistance. Nat. Biomed. Eng. 1, 0005 (2016).



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in