A sustainable paper-based platform for high-throughput 3D tumor models for ethical preclinical testing
Published in Biomedical Research
3D disease models are rapidly becoming indispensable tools in cancer research, offering more realistic tissue-like behavior than traditional 2D cell cultures and helping scientists better predict therapeutic responses [1]. As the global demand for physiologically relevant models continues to rise [2], researchers face major challenges not only in fabricating large numbers of these models and maintaining their consistency, but also in preserving them for future use, an ability that has remained largely out of reach for most 3D systems.
Unlocking cryopreservation for 3D grown tumor models would dramatically shorten preclinical testing timelines and improve drug-development outcomes by allowing laboratories to biobank ready-to-use tumor models instead of regenerating them repeatedly. A research team at NYU Abu Dhabi, led by Prof. Mohammad A. Qasaimeh, has now introduced SpheroMatrix [3], a simple, scalable, and cryopreservable platform for producing dense arrays of 3D tumor models using wax-patterned filter paper, potentially offering a fast, affordable, and reliable system for high-throughput drug screening and personalized medicine (Figure 1). This work builds directly on the group’s prior advances in paper-based cell cryopreservation [4], the establishment of standardized cryopreservation protocols [5], and the development of paper-based microarray platforms for 3D tumor model generation [6], providing a unified and extensible framework for scalable 3D culture and long-term biobanking.
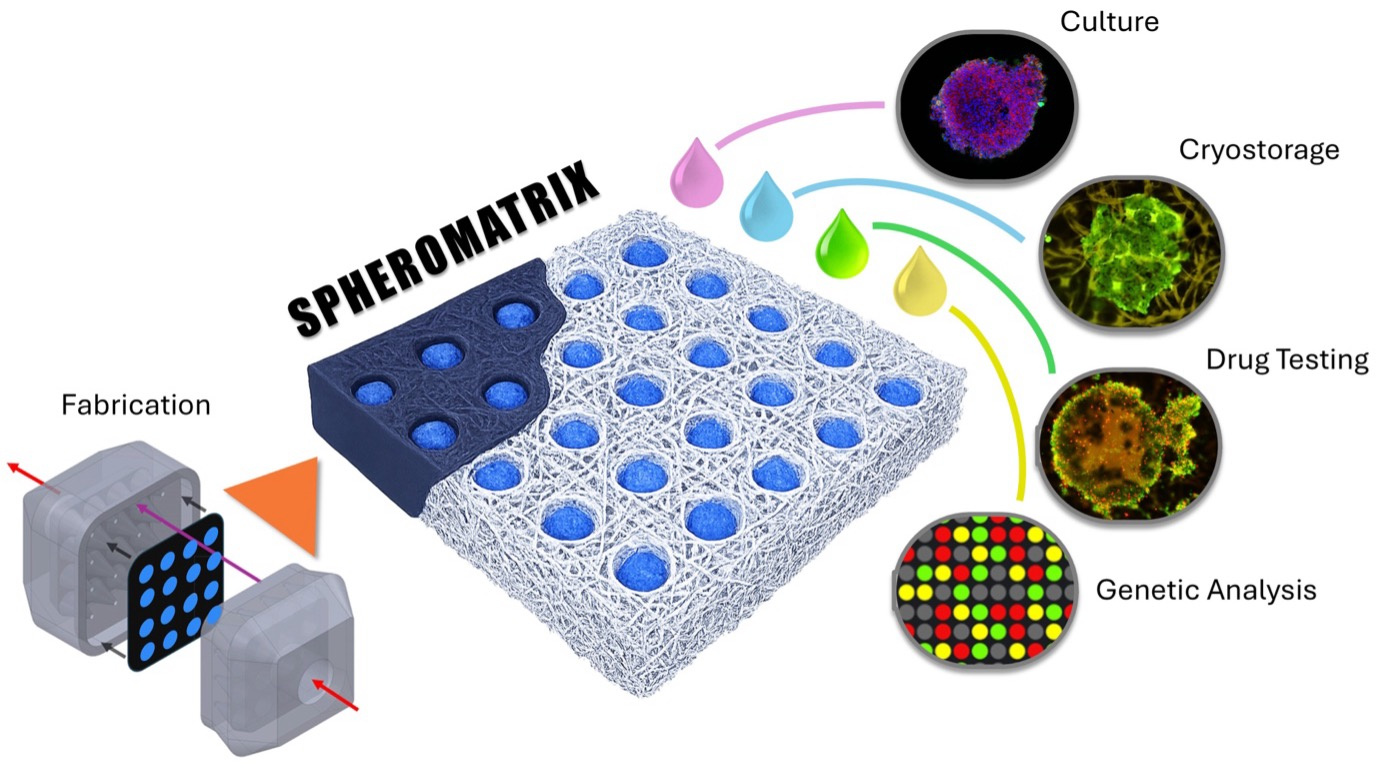
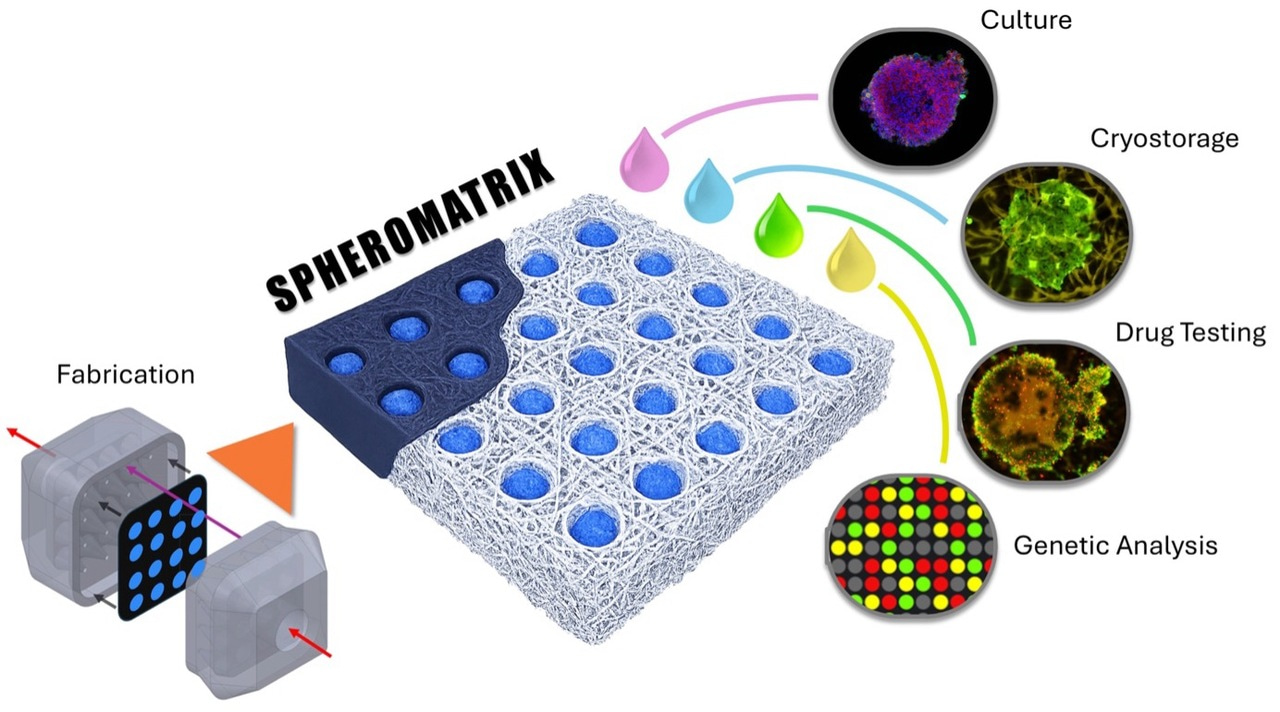
Figure 1. Paper-based Spheromatrix enables scalable 3D spheroid generation and cryopreservation while preserving viability, proliferation, and metabolic activity. Its workflow spans paper-based wax microwell fabrication and cell seeding, spheroid culture, cryopreservation, and post-thaw drug testing and genomic analysis.
Tumor model generation, cryopreservation, and drug testing.
To demonstrate its versatility, the SpheroMatrix was used to generate tumor models from six cancer cell lines and a 3D culture of one non-cancerous fibroblast line, capturing a broad spectrum of tumor behaviors ranging from low-metastatic breast cancer (MCF7) to aggressive glioblastoma (U87) and highly proliferative cervical cancer (HeLa) (Figure 2). Despite their biological differences, all cancer cell types successfully formed compact, metabolically active tumor models within the paper-based wax U-wells, each displaying characteristic growth patterns that matched their known clinical phenotypes. Even MRC5 fibroblasts, which are cells that typically struggle to form 3D aggregates due to their strong dependence on extracellular matrix adhesion, were able to assemble into viable 3D clusters, highlighting the platform’s adaptability. Longitudinal measurements revealed consistent trends in viability, proliferation, and metabolic activity across cell lines, with most cancer models maintaining or recovering viability above 90% over several weeks. The platform also reproduced expected drug-response behaviors: tumor models treated with cisplatin showed time-dependent reductions in metabolic activity similar to those generated in standard U-bottom plates, confirming the translational relevance of the paper-based system. Notably, SpheroMatrix tumor models, took one week to recover after thawing, retained their metabolic function and drug sensitivity, even after cryopreservation, demonstrating that the platform not only supports reliable 3D growth but also preserves biological integrity for long-term storage and downstream therapeutic testing.
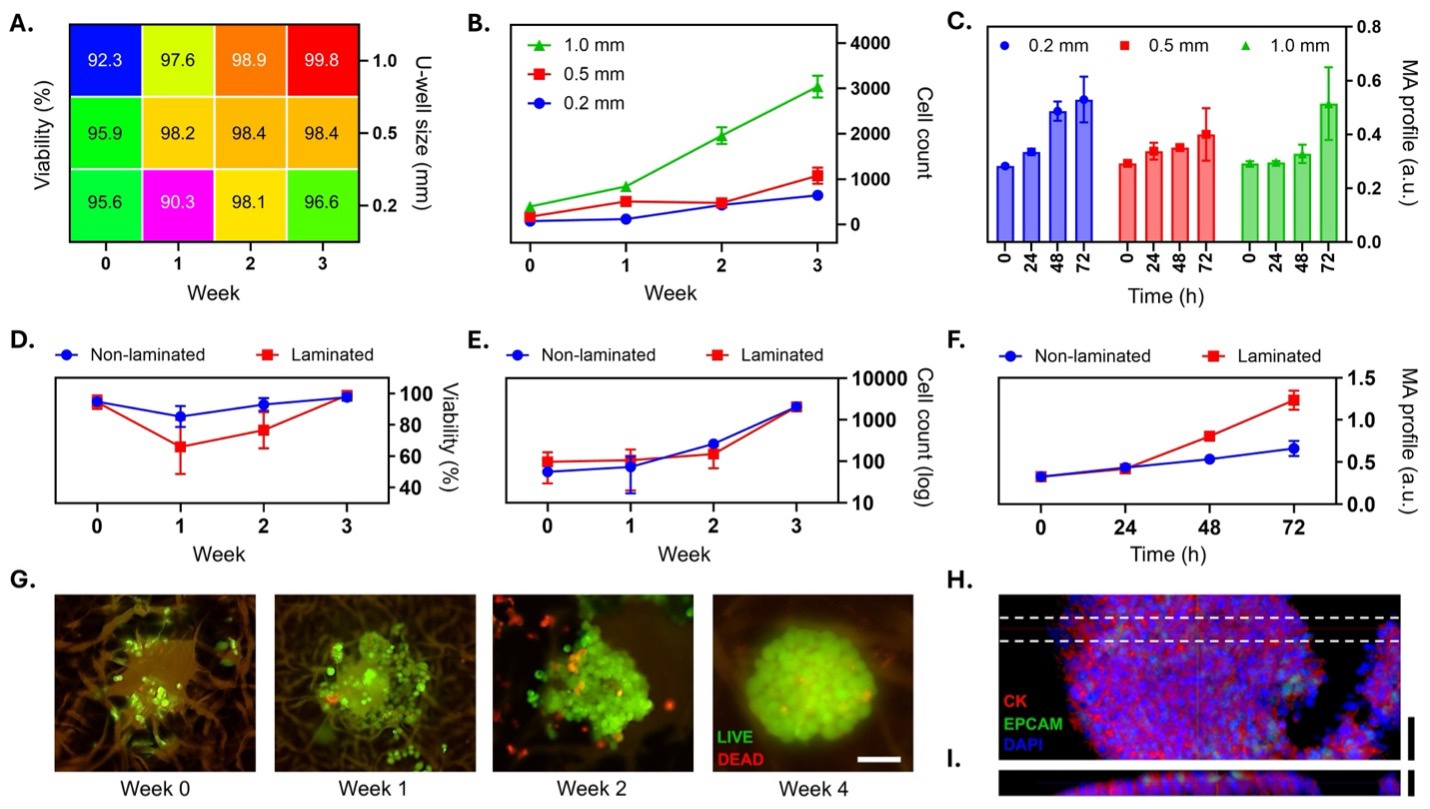
Figure 2. Spheromatrix-based spheroid formation and metabolic activity. (A) Heatmap of spheroid viability (%) in paper-wax U‐wells with diameters of 0.2, 0.5, and 1.0 mm, tracked over 3 weeks, indicating consistently high viability. (B) Cell growth curves show the strongest proliferation in 1.0‐mm U‐wells, whereas the smallest U‐wells show the slowest growth. (C) Metabolic activity (MA) measurements over 72 h confirm that larger U‐wells (1.0 mm) sustain higher metabolic activity compared to smaller U‐wells. n = 281 for panels (A–C). (D) Laminated versus non‐laminated U‐wells: The laminated group shows an initial dip in viability during early culture, followed by subsequent recovery. (E) Cell growth analysis reveals slower initial proliferation in non‐laminated wells, but both groups achieve comparable densities by Week 3. (F) Metabolic activity measurements reveal increase in laminated wells over time. n = 24 for panels (D–F). (G) Live/dead staining of MCF 7 cells confirms robust spheroid formation, with predominantly viable cells observed by Week 4. Scale bar is 100 μm. (H) Immunofluorescence staining shows cytokeratin (red), EpCAM (green), and DAPI (blue), demonstrating epithelial integrity and cohesive spheroid structure. (I) Cross‐sectional imaging reveals densely packed spheroids within the U‐well (dashed lines in H). Scale bars are 150 μm.
Advantages of the Spheromatrix Platform
A key advantage of the Spheromatrix is its sustainability and efficiency, offering a practical solution for laboratories, pharmaceutical companies, and cell banks seeking to generate large numbers of 3D tumor models while minimizing waste and resource use. Because the platform is built from inexpensive filter paper and a reusable, fully recyclable Spherobox, it dramatically reduces reliance on plastic consumables typically required for spheroid culture, and it uses far smaller volumes of media and reagents than standard well plates. This reduction in materials not only lowers experimental costs but also contributes to environmentally conscious research practices.
Beyond Cancer
Beyond cancer research, the SpheroMatrix platform is well suited for a wide range of tissue-modeling applications. Its gentle, paper-based microenvironment supports tissue engineering studies, co-culture systems, and the creation of complex microtissues that better reflect in vivo biology. The platform can also potentially offer promising alternatives for cosmetics and dermatology testing, providing reproducible, ethical 3D tissues without relying on animal models. With its modular design and ability to integrate diverse cell types and biochemical cues, SpheroMatrix serves as a foundational step toward future organ-on-a-paper systems for more advanced preclinical testing.
Advancing preclinical testing
The ability to create physiologically relevant 3D tumor models in a high-throughput configuration helps shorten preclinical testing timelines by eliminating the need to repeatedly regrow tumor models for each set of tests. By more closely mimicking the structural complexity, drug penetration patterns, and microenvironmental cues of real tumors, i.e. features that 2D cultures simply cannot replicate, the Spheromatrix can enable more predictive and reliable drug-evaluation workflows, ultimately improving the translational value of preclinical studies.
Conclusion
The Spheromatrix represents a meaningful step for laboratories which struggle generate, preserve, and use 3D tumor models [7]. By combining paper-based engineering with a recyclable 3D-printed delivery platform [3, 8], it offers an accessible, scalable, and environmentally conscious alternative to conventional spheroid platforms. Its ability to reliably produce physiologically relevant tumor and tissue models, and to store them long-term through cryopreservation, can streamline preclinical research, improve drug-screening accuracy, and accelerate discoveries across oncology, tissue engineering, and regenerative medicine. As laboratories continue to shift toward more predictive and ethical testing systems, the Spheromatrix stands out as a practical tool that brings high-quality 3D culture capabilities within reach of researchers everywhere.
References
- Skardal, A., et al., Substrate elasticity controls cell proliferation, surface marker expression and motile phenotype in amniotic fluid-derived stem cells. J Mech Behav Biomed Mater, 2013. 17: p. 307-316.
- Bissell, M.J., H.G. Hall, and G. Parry, How does the extracellular-matrix direct gene-expression? J Theor Biol, 1982. 99(1): p. 31-68.
- Glia, A., et al., Spheromatrix: a paper-based platform for scalable 3D tumor model generation, cryopreservation, and high-throughput drug assessment. Microsystems & Nanoengineering, 2025. 11(1): p. 219.
- Alnemari, R., et al., Paper‐Based Cell Cryopreservation. Advanced Biosystems, 2020. 4(3): p. 1900203.
- Deliorman, M., et al., A method to efficiently cryopreserve mammalian cells on paper platforms. Bio-protocol, 2020. 10(18): p. e3764-e3764.
- Samara, B., et al., Cryopreservable arrays of paper-based 3D tumor models for high throughput drug screening. Lab on a Chip, 2021. 21(5): p. 844-854.
- Lee, K.H. and T.H. Kim, Recent advances in multicellular tumor spheroid generation for drug screening. Biosensors, 2021. 11(11): p. 445.
- Qasaimeh, M.A., Advances in microfluidic automation and manipulation. IEEE Nanotechnol. Mag., 2020. 14(3): p. 5.
Follow the Topic
-
Microsystems & Nanoengineering

This journal, with a target for a high-end journal for years to come, seeks to promote research on all aspects of microsystems and nanoengineering from fundamental to applied research.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in