A wearable autonomous biomimetic sensor for precision nutrition
Published in Bioengineering & Biotechnology

Most people will be as frustrated as we are when asked to make frequent hospital visits or take multiple blood draws a day. Nearly four years ago, we were conceiving ideas to develop wearable telemedicine sensors to monitor an individual’s health state continuously and noninvasively, and to enable timely intervention under home- and community-based settings.
With such a question in mind, we quickly formed a small team and cast our eyes on sweat, sweat is an important body fluid containing a wealth of chemicals reflective of nutritional and metabolic conditions, which can be used as a medium to indirectly reflect health status. For example, our previous experimental results found that the sweat uric acid levels in gout patients are higher than those in healthy subjects, and that the sweat uric acid level after medication for a patient is much lower than before medication. The pilot study of sweat-serum uric acid correlation shows the potential of using sweat uric acid as a biomarker for gout monitoring and potentially therapeutic evaluation (for details, please see Nature Biotechnology 38, 217–224 (2020)).
The next question was to construct a biomarker detection method that can monitor many other biomarkers and meet the requirements of high stability, high accuracy, and long-term cycle detection. Common biocomponents such as antibodies or enzymes have been used as specific recognition elements in the fabrication of biosensors. However, these sensors have inherent limitations such as vulnerability to interference from other ions present in the sample solutions and lack of selectivity to a particular analyte even if high-affinity biomolecules are used. Given the often poor chemical and physical stability of these biomolecules, which is especially problematic in wearable devices. Comparing a variety of currently reported biomarker detection methods, we found that molecularly imprinted polymers (MIPs) have earned the reputation as ‘artificial antibodies.’ MIPs possess several distinct advantages over other receptors: high selectivity and affinity, desirable chemical and physical stabilities, ease of adaptation to practical applications, and versatility in imprinting several a wide array of targets including small molecules, peptides, proteins, and cells. Through the optimization of MIP synthesis parameters along with the theoretical calculation, we successfully constructed a series of ‘artificial antibodies’ that can detect individual amino acid molecules with a simple electro-polymerization method. Considering that a total level of multiple nutrients is often an important health indicator, a multi-template MIP approach has also been used to enable accurate and sensitive detection of the total concentration of multiple targets with a single sensor. Interestingly, the constructed MIP sensors can be regenerated in situ upon applying a constant potential to the working electrode that repels the bound target molecules from the MIP layer with prolonged re-usability.
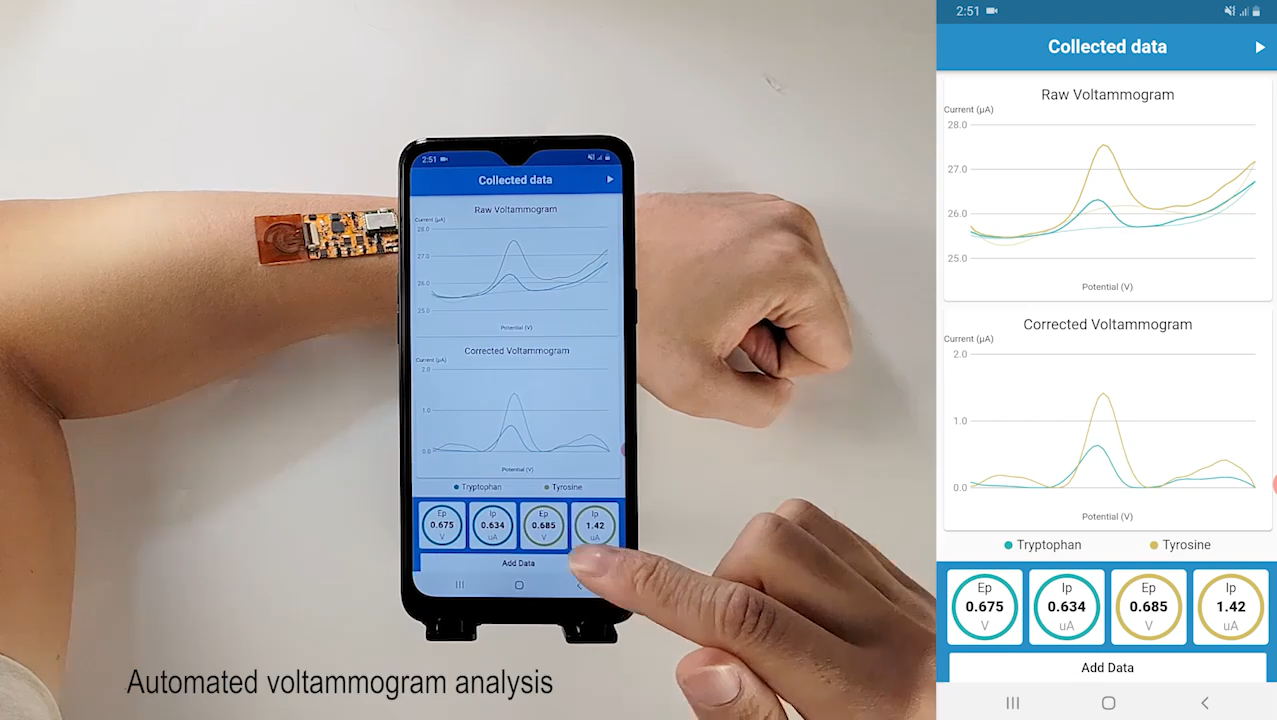
The next challenge was to apply this detection method to practical applications and meet the requirements of wearable, real-time, and continuous in-situ monitoring of health biomarkers. Through a strategic integration of our previously reported mass-producible laser-engraved graphene (LEG), redox-active nanoreporters, MIP-based ‘artificial antibodies’, along with in situ regeneration technologies, we developed a universal sensing strategy toward continuous analysis of a number of trace-level metabolites and nutrients including all essential amino acids and vitamins. Seamless integration of this technology with in situ signal processing and wireless communication leads to a powerful wearable sweat sensing platform ‘NutriTrek’ that is able to perform personalized and non-invasive metabolic and nutritional monitoring toward timely intervention.
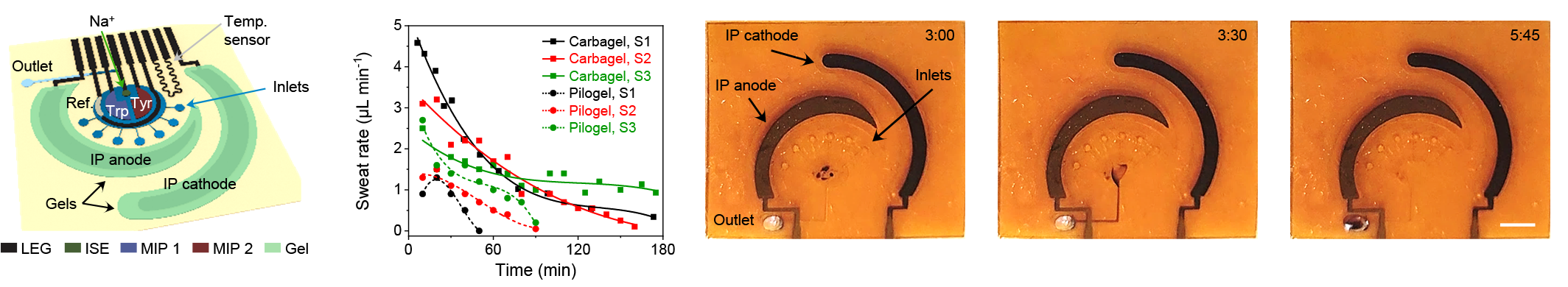
To enable on-body continuous metabolic and nutritional monitoring, the flexible sensor patch was designed to comprise of an iontophoresis module for localized on-demand sweat induction, a multi-inlet microfluidic module for efficient sweat sampling, a multiplex LEG-MIP sweat nutrient sensor array for continuous amino acid (AA) analysis, and LEG-based temperature and electrolyte sensors for real-time AA sensor calibration. In order to make this wearable technology broadly applicable, particularly for sedentary individuals, we utilized a custom-designed iontophoresis module consisting of the LEG anode and cathode coupled with hydrogels containing muscarinic agent carbachol (carbagel) for sustainable sweat extraction. Carbachol was selected from various muscarinic agents as it allows the most efficient, repeatable, and long-lasting sweat secretion from the surrounding sweat gland thanks to its additional nicotinic effects. With the optimized design for sweat induction and sampling, sweat can be conveniently induced locally and readily sampled. In collaboration with nutritionists, we are evaluating our wearable systems for prolonged metabolic and nutritional monitoring across activities, during physical exercise and at rest. Through multiple human studies, we demonstrate the platform’s high potentials toward real-time monitoring of dietary nutrient intakes, central fatigue, risks of metabolic syndrome, and COVID-19 severity. Right now, we are developing more methods to conveniently fabricate and integrate biosensors for monitor a broad range of nutrients, metabolites, and cancer drugs, and evaluate our sensors in Long-COVID and patients with metabolic syndrome.
In summary, we developed integrating fundamental chemistry and materials innovations with practical device and system level applications toward personalized metabolic and precision nutrition. With the continuing technical innovations and working closely with our clinical nutrition collaborators, we believe our wearable biosensing technology could play a crucial role in future personalized metabolic and nutritional management.
These results were recently published in Nature Biomedical Engineering: M. Wang, Y. Yang, J. Min, Y. Song, J. Tu, D. Mukasa, C. Ye, C. Xu, N. Heflin, J. S. McCune, T. K. Hsiai, Z. Li, W. Gao*, A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients, Nature Biomedical Engineering, 2022, 10.1038/s41551-022-00916-z.
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in