Advancing Azapeptides- A novel platform to expedite azapeptide synthesis and accelerate therapeutics development
Published in Protocols & Methods

Peptides are increasingly used as therapeutic agents and offer the advantage of increased surface area for disruption of protein-protein interactions or agonist-like binding, which can help to improve specificity and limit off target effects. Their main weakness has been rapid metabolism by peptidases/proteases, which can limit their therapeutic relevance (dosing schedule and bioavailability). Peptidomimetics, which incorporate non-natural components into the amino acid chain, have been developed to help overcome some of the therapeutic restraints of natural peptides. Azapeptides are one type of peptidomimetic which contain one or more aza-amino acid residues (alpha carbon replaced by a nitrogen) and this feature has the potential to bestow resistance to endogenous enzyme metabolism, while also preserving main features of the natural peptide. Synthesis of azapeptides has previously been reserved for specialized chemistry labs, necessitating custom syntheses of each azapeptide analogue, and also relying on methods that were inefficient.
It was our opinion that the field of azapeptides synthesis should be easily incorporated into existing peptide synthesis techniques, thus there was a need for a platform of reagents, and methods of activation that enable peptide chemists to synthesize azapeptides and libraries thereof, using standard peptide synthesizer instruments. As such a platform did not exist, we engineered a more universal method for azapeptide synthesis that was compatible with conventional peptide synthesis methodology. Employing a thiocarbazate scaffold, we developed a series of sixteen analogues and associated activation methods which can be used to create custom azapeptides using standard solid phase peptide synthesis (SPPS) instruments.
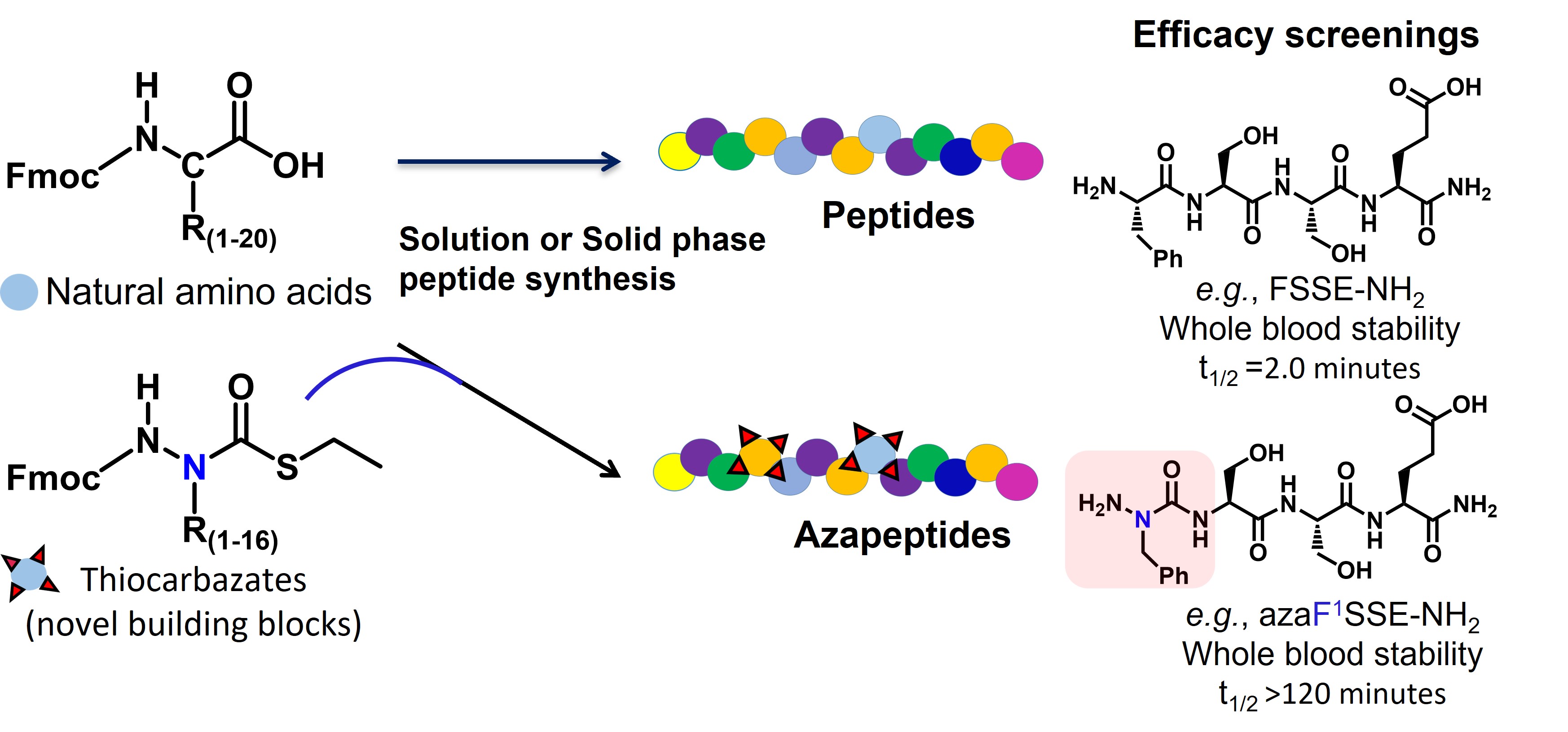
Figure. Patented thiocarbazate building blocks (based on 16 amino acids) can be added in strategic locations to build potential therapeutic peptides which can be screened for desired characteristics.
We have patented these unique thiocarbazate building blocks and associated methods and look forward to partner with academia and pharma companies to accelerate the field of therapeutic azapeptide development. In order to demonstrate the robustness of our system and methods we created targeted libraries of azapeptides around two peptides, FSSE/P5779- an HMGB1 (high-mobility group box-1) pathway antagonist, and bradykinin- an endogenous bioactive peptide. Using successive screening of library candidates, starting with ex vivo whole blood half-life, we selected lead azapeptide candidates from each peptide target and tested in both in vitro and in vivo efficacy studies to discover lead azapeptide therapeutic candidates for both peptide targets. These proprietary azapeptide building blocks and methods, combined with efficacy screening can be used to rapidly and efficiently select and develop leading azapeptide-based therapeutics. You can read more about our approach and methods in the paper in Nature Communications, https://www.nature.com/articles/s41467-022-34712-9
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in