Advancing Health Equity for Medical Devices
Published in Healthcare & Nursing
By Kushal T. Kadakia, Vinay K. Rathi, Joseph S. Ross, Sanket S. Dhruva
Health inequity describes differences in access to care and health outcomes due to living conditions, social factors and processes, and axes of identity.1 While the causes and manifestations of such inequities are multifaceted, a substantial body of evidence has already demonstrated the relationship between health disparities and worsened disease incidence, care quality, clinical outcomes, and life expectancy.2 Efforts to address health inequities are therefore both a policy priority and moral imperative.
One manifestation of inequity in the health care system is the uneven distribution of innovations in medical technology, specifically medical devices. Medical devices play an integral role in medicine, supporting diagnosis, decision-making, and treatment. From the advent of technologies like transcatheter heart valves for aortic stenosis, to the development of new imaging platforms powered by artificial intelligence, innovation in medical technology has significant potential to play a key role in patient care and human health. However, there are notable disparities in access to medical devices, which in some cases are a byproduct of the systemic inequities that pervade the health care system.
For example, ischemic stroke care has historically relied on clot-dissolving medications to restore blood flow to the brain. Over the past decade, physicians have increasingly begun using new device-enabled treatment approaches as adjuncts to (and in some case, substitutes for) medications for stroke treatment. In these mechanical thrombectomy procedures, physicians deploy medical devices to locate the blockage and manually remove the clot to re-establish cerebral blood flow. Though clinical research demonstrates convincing improvements in brain function after thrombectomy, patients living in rural areas are less likely to receive this treatment due to geographic barriers to access.3 Moreover, white and non-Hispanic patients are more likely to be transferred to a hospital that offered mechanical thrombectomy.4
At the same time, emerging scholarship documents how the performance of some medical device technologies varies across different populations, with implications for clinical outcomes. Consider the case of pulse oximeters, which doctor’s offices and hospitals widely use to estimate the amount of oxygen in patient’s blood (“SpO2”) based on the relative amount of light absorption. These estimates critically inform management decisions, such as whether patients with COVID-19 receive supplemental oxygen or antiviral therapy. However, recent research has revealed that pulse oximeter devices often provide inaccurate SpO2 estimates for darker-skinned individuals, potentially delaying the initiation of necessary medical care for racial and ethnic minority patients.5
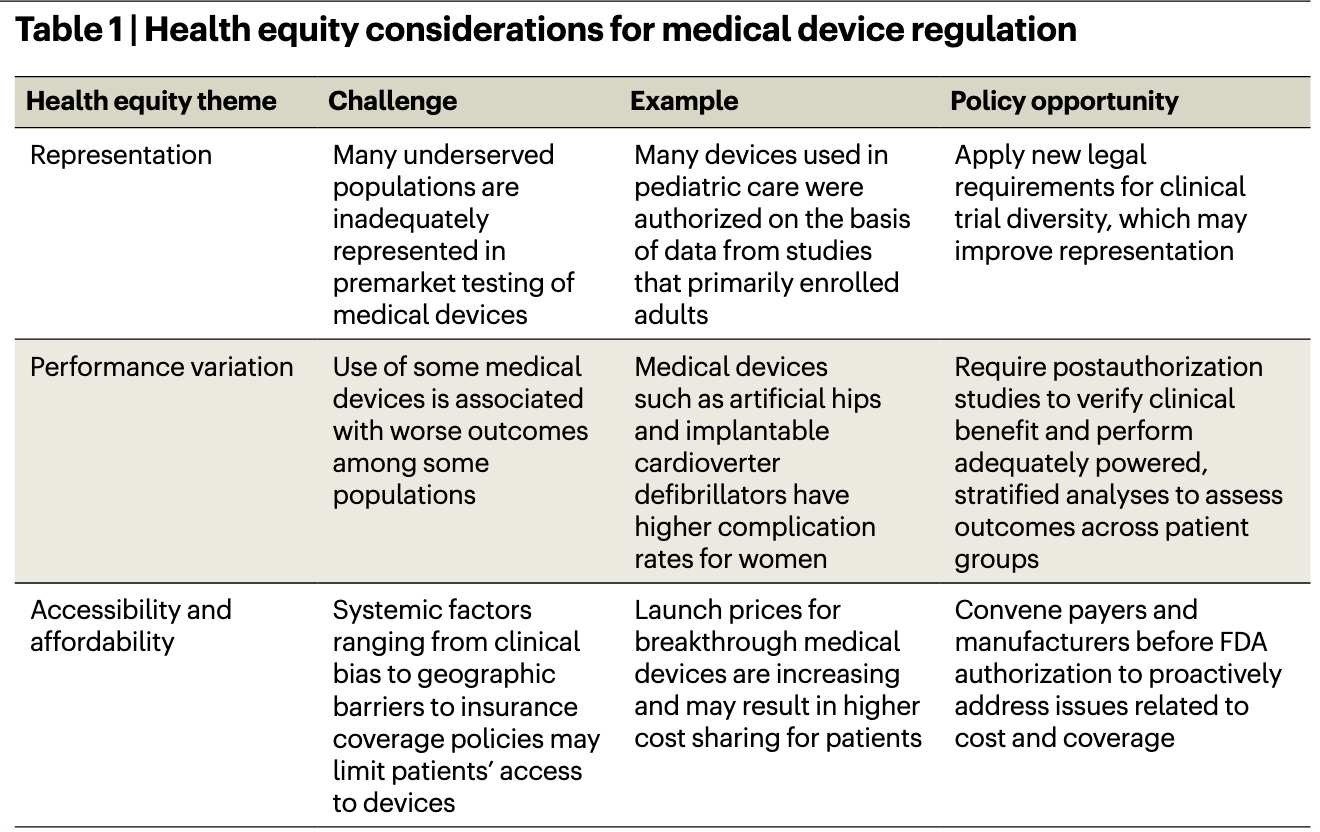
These examples illustrate the need for reforms that advance health equity during medical device development. In our new article published in Nature Biotechnology, we explore the core equity considerations for medical device regulation, including clinical trial representation, performance variation, and accessibility and affordability (Table).6 We examine these themes in the context of a recent proposal from the Food and Drug Administration (FDA) to embed equity considerations into the agency’s Breakthrough Devices Program (BDP).
The BDP aims to expedite development and review for medical devices that diagnose or treat life-threatening conditions. Devices that receive a “Breakthrough” designation receive priority review, may require less rigorous evidence for authorization, and automatically qualify for additional reimbursement from Medicare, which insures over 65 million patients in the U.S. Between 2016 and 2022, FDA authorized 62 Breakthrough devices and awarded Breakthrough status to an additional 698 more devices that could receive authorization in the future.7
In fall 2022, the FDA proposed expanding BDP eligibility to include devices focused on health equity, asserting that such devices are consistent with the program’s focus on technologies that offer “more effective treatment or diagnosis”.8
While the policy is well-intentioned, it neither addresses the systemic factors responsible for disparities in medical device development, nor does it include the necessary guardrails to ensure that such devices actually deliver on manufacturer claims of advancing equity. Furthermore, the existing shortcomings of the BDP may prove particularly harmful for equity-focused devices. For example, researchers have raised concerns about the adequacy of premarket testing for Breakthrough devices and potential cost implications for patients.9 These same issues could prove particularly problematic for equity-focused Breakthrough devices by exposing vulnerable populations to devices authorized despite less rigorous evidence and/or priced at a premium that causes undue financial burden.
In our article, we contextualize the draft reforms to the BDP within the broader disparities associated with medical device regulation, and offer recommendations to strengthen FDA’s proposed policy. We believe that using an “ends-justifies-the-means” approach to regulation is ill-suited for advancing the goal of health equity. Just because a device claims to address disparities does not guarantee that it will; verifying this claim requires evidence generated from rigorous studies that enroll diverse populations. However, the draft policy does not specify what requirements for premarket or postmarket evidence generation are needed for disparities-focused devices.
The FDA’s proposal could be strengthened by clarifying expectations for evidence, including requiring confirmatory studies to verify clinical benefit and disparities reductions. If a device’s claim of equity cannot be verified in follow-on studies, then FDA could consider rescinding its Breakthrough designation. FDA could also consider coordinating with payers to ensure device accessibility and affordability, with a focus on ensuring coverage for patients of low socioeconomic status and limiting out of pocket costs associated with Breakthrough devices. The agency could also explore opportunities to advance equity for all medical devices, regardless of Breakthrough designation.
Reforms focused on the systemic drivers of inequity can help redesign regulation to advance health equity for all. Inequitable access to effective and safe medical devices is but one of many areas that may benefit from focused attention from policymakers. Revising FDA’s disparities proposal for Breakthrough devices can serve as a starting point for broader reforms focused on improving access to innovative technologies so that all patients have the potential to achieve improved health.
References
- The World Health Organization. Health Equity. https://www.who.int/health-topics/health-equity (2023).
- Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. https://nap.nationalacademies.org/catalog/12875/unequal-treatment-confronting-racial-and-ethnic-disparities-in-health-care (2003).
- Kamel, H. et al. Access to Mechanical Thrombectomy for Ischemic Stroke in the United States. Stroke 52, 2554-2561 (2021).
- Rinaldo, L. et al. Racial and Ethnic Disparities in the Utilization of Thrombectomy for Acute Stroke: Analysis of Data From 2016 to 2018. Stroke 50, 2428-2432 (2019).
- Fawzy, A. et al. Racial and Ethnic Discrepancy in Pulse Oximetry and Delayed Identification of Treatment Eligibility Among Patients With COVID-19. JAMA Intern Med 182, 730-738 (2022).
- Kadakia, K. et al. Challenges and Solutions to Advancing Health Equity with Medical Devices. Nature Biotechnology (2023).
- Center for Devices and Radiological Health 2022 Annual Report. https://www.fda.gov/about-fda/cdrh-reports/cdrh-2022-annual-report (2023).
- S. Food and Drug Administration Draft Guidance: Select Updates for the Breakthrough Devices Program Guidance: Reducing Disparities in Health and Health Care. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/select-updates-breakthrough-devices-program-guidance-reducing-disparities-health-and-health-care (2022).
- Johnston, J. et al. Early Experience with the FDA’s Breakthrough Devices Program. Nature Biotechnology 38, 933-938 (2020).
Follow the Topic
-
Nature Biotechnology

A monthly journal covering the science and business of biotechnology, with new concepts in technology/methodology of relevance to the biological, biomedical, agricultural and environmental sciences.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in