Aging as the Wound That Fails to Heal — A Bioenergetic Continuum of Resolution Failure
Published in Cell & Molecular Biology and Paediatrics, Reproductive Medicine & Geriatrics
When we first encountered Ogrodnik’s “wound that never heals” model of aging, the metaphor resonated deeply. It captured something we frequently see in clinical practice: individuals who are not “sick” in the traditional sense, yet fail to recover, regenerate, or bounce back from stressors that once posed no challenge. Their bodies behave like wounds that do not quite complete healing.
But a central question remained unanswered:
Why does healing stall in aging—and what determines whether tissues successfully transition from inflammation to regeneration?
This Perspective arose from years of trying to answer that question through clinical observation, mechanistic synthesis, and the development of the Exposure-Related Malnutrition (ERM) framework.
Where the Idea Began: The Missing Link in Aging Theory
Modern geroscience offers multiple powerful frameworks—the hallmarks of aging, hormesis, mitochondrial dysfunction, antagonistic pleiotropy. Yet each describes pieces of the puzzle. We found that none of these fully explained a recurring phenomenon:
Aging is not simply the accumulation of damage, but the failure to complete resolution.
At the bedside, we often see stalled recovery long before traditional biomarkers become abnormal. Muscle injuries repair more slowly. Inflammation lasts longer. Fatigue becomes persistent. Metabolic inflexibility emerges. These patterns seemed less like “wear and tear” and more like a progressive shift in energy allocation.
This is where ERM entered.
ERM began as a way to explain subtle metabolic decline in chronic stress and non-communicable diseases. But it soon became clear that the same principles—substrate misallocation, mitochondrial congestion, redox imbalance, NADPH scarcity—could help explain why aging tissues cannot re-enter the anabolic phase of repair.
This paper represents the first attempt to unify these influences into a bioenergetic continuum of resolution failure.
The Conceptual Breakthrough: Aging as a Bioenergetic Problem
The transformative idea driving this Perspective was simple:
Healing requires energy. Not just ATP, but the right kind of energy, in the right place, at the right time.
Across molecular, cellular, and systemic scales, we found that:
-
Regeneration requires BOTH ATP and NADPH.
-
NADPH—rarely discussed in geroscience—is essential for collagen synthesis, antioxidant defense, and redox balance.
-
Stress responses are metabolically expensive, draining the same substrate pools needed for repair.
-
Mitochondrial “dysfunction” is often an adaptive, reversible slowdown—not a primary defect.
But most importantly:
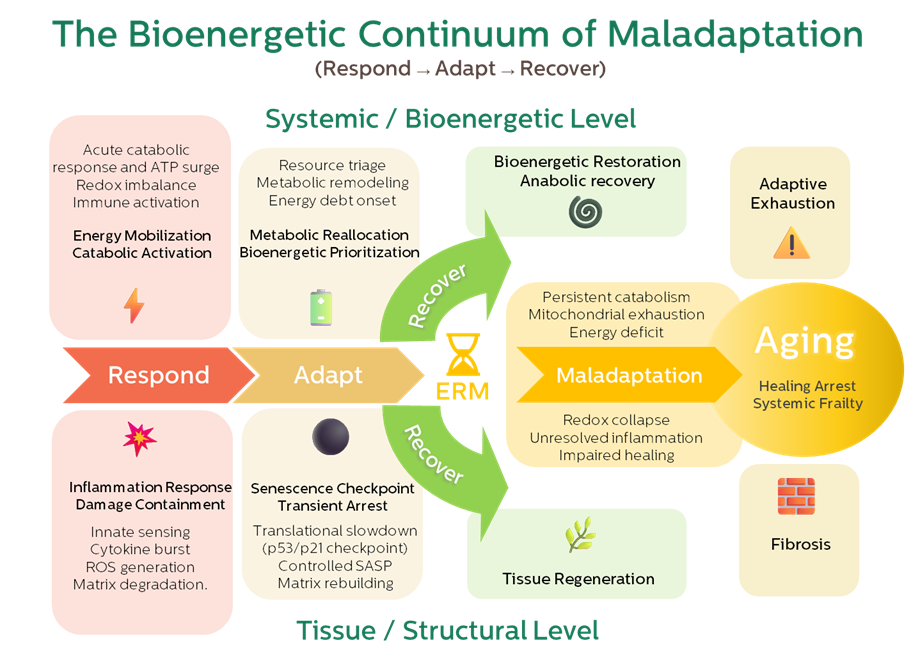
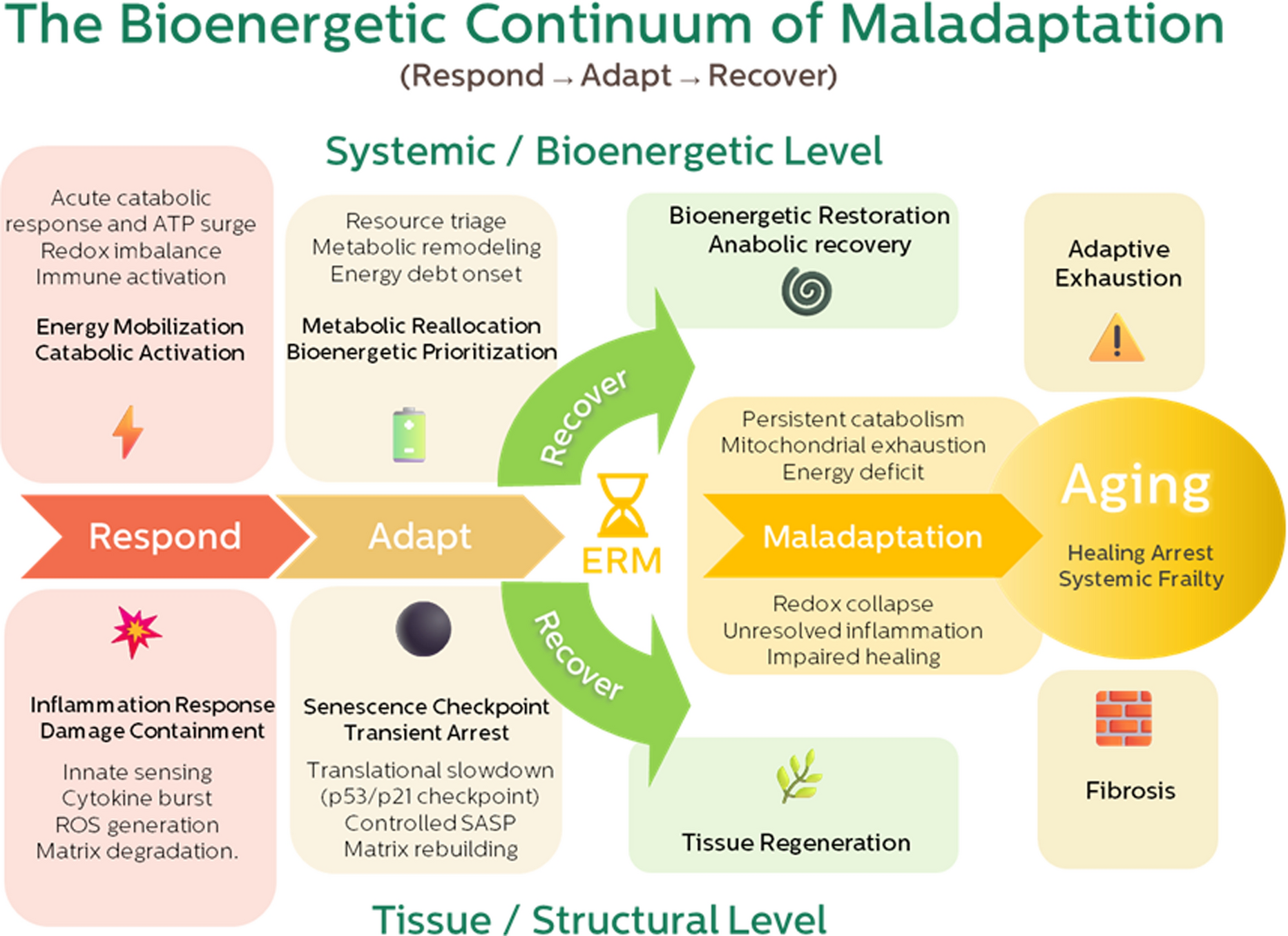
Aging begins when the transition from catabolic response to anabolic restoration fails—not because repair programs vanish, but because the energy required to run them is no longer available.
This became the foundation of our “bioenergetic continuum.”
The figure in the paper (Fig. 1) visually captures this evolution: from Respond → Adapt → Recover, and how the inflection point we call ERM marks the moment when recovery becomes constrained.
The Surprising Role of Lipid Droplets and Metabolic Gridlock
One of the most unexpected insights came from synthesizing literature on lipid droplets (LDs). Traditionally viewed as neutral storage, LDs are now recognized as markers of metabolic stress and innate immune activation.
What we found is that:
-
LD accumulation is an adaptive attempt to protect the cell, sequestering excess fatty acids and preventing redox collapse.
-
But chronically, LDs represent an energy misallocation signal—a shift away from oxidative repair toward survival-first glycolysis.
-
This same metabolic pattern appears in aging tissues, chronic wounds, and persistent inflammation.
This reinforced the central thesis:
Aging is not a molecular mystery—it is a metabolic bottleneck.
Reframing Mitochondrial Dysfunction
Perhaps the most contentious point in the field is whether mitochondrial dysfunction is a cause or consequence of aging.
Our synthesis suggests something in between:
-
Mitochondria downregulate oxidative flux adaptively under stress to preserve redox balance.
-
This adaptive slowdown only becomes pathological when chronic substrate excess, NADH overload, or acetyl-CoA inflexibility produces gridlock.
-
Thus, mitochondrial dysfunction is both:
-
A protective response, and
-
A marker of unresolved recovery.
-
This view bridges classical damage-centered theories with newer stress-adaptive perspectives.
Senescence Reconsidered: A Terminal Energetic Checkpoint
We argue that senescence represents the terminal expression of sustained bioenergetic scarcity.
This does not diminish its genomic or tumor-suppressive role—it contextualizes it. When ATP and NADPH fall below recovery thresholds:
-
cells cannot safely divide,
-
acetyl-CoA overflow stabilizes stress transcription,
-
NAD⁺ decline locks chromatin in an inflammatory state,
-
the AMPK–mTOR cycling rhythm collapses.
Senescence becomes the biological equivalent of a power outage: the cell shuts the door on proliferation to preserve itself.
We believe viewing senescence through this energetic lens helps reconcile why:
-
senolytics improve regeneration but sometimes impair healing,
-
caloric restriction extends lifespan despite “less energy”,
-
early immunosenescence can be reversible when energetic balance is restored.
What This Means for the Future of Geroscience
This Perspective proposes that the next frontier in aging research lies in mapping the energetic thresholds that determine whether tissues:
-
recover,
-
stall,
-
or enter irreversible decline.
We suspect key biomarkers—ATP flux, NAD⁺/NADH turnover, NADPH production, cf-mtDNA, GDF15—will become essential to quantify this landscape.
Ultimately, we believe aging research will increasingly focus on:
-
restoring fuel-selection flexibility,
-
relieving substrate congestion,
-
re-opening mitochondrial–epigenetic communication, and
-
recovering the rhythmic cycling between catabolism and anabolism.
The future of geroscience will be defined not just by lowering damage, but by restoring energy for resolution.
A Personal Note
This paper grew out of years of clinical encounters—patients stuck in recovery, whose symptoms resembled low-grade, unresolved healing. Their stories pushed us to ask whether aging itself could be understood as failed recovery rather than inevitable decline.
We hope this Perspective inspires researchers, clinicians, and students to explore aging not only as a molecular phenomenon, but as a dynamic story of energy, adaptation, and resilience.
Reference:
Tippairote, T., Hoonkaew, P., Suksawang, A., & Tippairote, P. (2026). Aging as the wound that fails to heal: a bioenergetic continuum of resolution failure. Biogerontology, 27:7.
Follow the Topic
-
Biogerontology

Biogerontology is a peer-reviewed journal dedicated to exploring the biological basis and mechanisms of ageing, with an aim of promoting healthy old age.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in