Mercury, the Gut, and Parkinson’s Disease—A Case Series from Thailand
Published in Neuroscience, Biomedical Research, and General & Internal Medicine
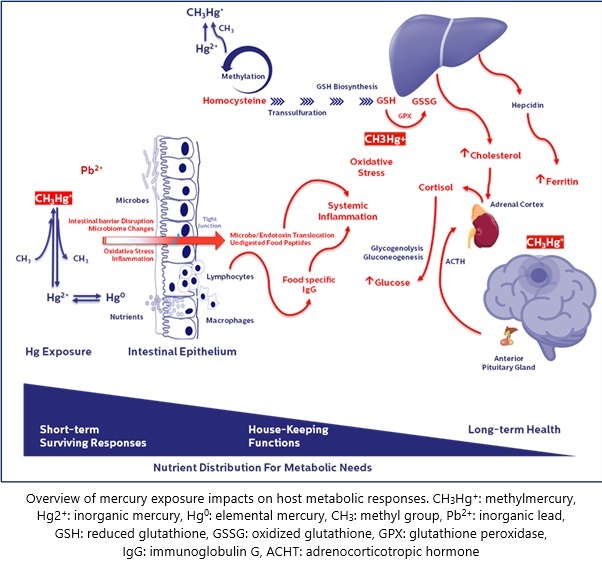
When we embarked on this case series, our goal was simple: to listen more deeply to the lived experiences of our Parkinson’s disease (PD) patients and investigate what might be hiding beneath the surface of conventional assessments. Over the years, we began noticing a recurring pattern—non-motor symptoms like gastrointestinal distress and fatigue often emerged before classic PD signs. These symptoms rarely received the clinical attention they deserved, but they sparked a question: Could there be an upstream trigger, particularly one involving environmental exposures?
In our recently published paper in SN Comprehensive Clinical Medicine, we explore a potential association between chronic low-level mercury exposure, gut barrier dysfunction, and PD progression. We examined three Thai male patients with idiopathic PD, each with elevated blood mercury levels and evidence of gut permeability dysfunction, as indicated by food-specific IgG patterns. The results, while preliminary, point to a potentially underrecognized pathway through which environmental toxins may exacerbate neurodegeneration.
What made these cases stand out?
Despite similar PD diagnoses, the patients’ clinical trajectories diverged sharply. One patient showed significant improvement with dietary changes and antioxidant support. Another remained stable, while the third experienced marked disease progression alongside persistently high toxin levels, chronic inflammation, and metabolic dysregulation. These differences suggest that allostatic load—the cumulative burden of stressors including toxins, inflammation, and metabolic imbalance—may influence how PD unfolds in each individual.
Challenges behind the scenes
One of the biggest hurdles we faced was the limited availability of specific gut barrier biomarkers in routine clinical settings. We had to rely on food-specific IgG panels as surrogate indicators—a strategy not without controversy, but useful in guiding elimination diets and exposure management. Another challenge was helping patients and caregivers navigate the emotional and logistical complexity of long-term dietary and lifestyle modifications, which often require sustained motivation and clinical support.
Why this matters
While mercury’s role in neurotoxicity is well established in high-dose exposures, its impact at chronic, low levels—especially when combined with other stressors like lead, metabolic imbalance, and gut dysfunction—is far less understood. Our findings add to the growing body of evidence that neurodegenerative diseases like PD may involve systemic and environmental contributors beyond dopamine deficiency. They also underscore the need for personalized medicine approaches that account for toxic burden, redox balance, and gut health.
Looking ahead
This case series is not the final word, but rather an invitation for deeper inquiry. We hope it inspires future research on toxin kinetics, gut-brain interactions, and precision care strategies in PD. If there's one thing our patients have taught us, it’s that resilience isn’t just about what we treat—it’s about understanding what each person is carrying.
Follow the Topic
-
SN Comprehensive Clinical Medicine

A broadly based, peer reviewed journal that publishes original research in all disciplines of clinical medicine and their subspecialties, including all aspects of Imaging, Surgical and Medical studies related to diagnosis, treatment and management.
Related Collections
With Collections, you can get published faster and increase your visibility.
Obesity and type 2 diabetes
According to the recent esteems, more than 800 million adults have type 2 diabetes (T2D), with an increase of about 600 million in the last 30 years. The largest increases are in low-income and middle-income countries. As a matter of fact, a parallel increase has been constantly reported for the prevalence of obesity and overweight. Obesity has increased to fewer than 2 billion in the last 5 years. Obesity was recognized as a disease by WHO about 80 years ago and very recently by the Italian Parliament as a chronic, progressive, and relapsing disease.
A multifactorial etiology (both genetic and environmental factor) has been proposed for T2D with two main pathogenetic mechanisms (the defect of insulin production and insulin resistance). The dysfunction of β-cells is commonly attributed to the loss of β-cell mass (by exhaustion) or apoptosis (by glucotoxicity and lipotoxicity). More complex mechanisms and interactions are involved, such as dedifferentiation of β-cells, oxidative stress, induction of disallowed genes, dedifferentiation, transdifferentiation, endoplasmic reticulum stress, altered prostaglandin signaling, mitochondrial dysfunction and amyloidosis. The insulin resistance (IR) is represented by the reduced response to insulin in the target tissues. Many hormones affect the action of insulin (classically, growth factors and insulin-like growth factor 1, glucagon, glucocorticoids, and catecholamines). Beyond the genetics, obesity, sedentary lifestyle, dietary habits, chronic stress and sleep deprivation can all negatively impact insulin sensitivity, and, recently, gut microbiota has been considered in the pathophysiology of T2D.
The signaling pathway of insulin may disrupted at different levels, from upstream (the insulin receptor) to downstream (for example, the glucose transporter or GLUT1-4).
In the development of T2D, extra-pancreatic factors are essential, such as the IR at the level of the skeletal muscles, the adipose tissue and the liver. The detrimental effects of IR virtually involve every tissue of the body that present the insulin receptor, and growing evidence in the literature support an important damage at the central nervous system.
The well-known complications of T2D are: diabetic kidney disease (DKD), diabetic retinopathy (DR), diabetic neuropathy (DN), metabolic dysfunction-associated steatotic liver disease (MASLD), cardiovascular disease (CVD) (coronary artery disease or CAD, stroke and peripheral artery disease or PAD).
Considering obesity, several districts of the body are affected by this disease, such as the respiratory system (apnoeas/hypopnoeas and hypoventilation during sleep), cardiovascular system (preserved or reduced left ventricular systolic function - heart failure, arrythmias and atrial fibrillation, systemic and pulmonary artery hypertension, aortic valve stenosis), lower limbs lymphedema, deep venous system and/or pulmonary thromboembolic disease, metabolism (hyperglycaemia and T2D, high triglyceride levels, and low HDL cholesterol levels, metabolic syndrome), the liver (MASLD and hepatic fibrosis), the kidney (microalbuminuria, reduced glomerular filtration rate), urinary incontinence, reproduction (anovulation, oligo-menorrhea and polycystic ovary syndrome, male hypogonadism), musculoskeletal system (osteoarthritis).
The recent Delphi Consensus defined obesity an excessive adiposity (based on recent anthropometric measures), with or without abnormal distribution or function of the adipose tissue, caused by multifactorial pathogenetic mechanisms (genetic, environmental, neurobiological, psychological, socioeconomic, nutritional and metabolic factors) and still incompletely understood. Obesity can cause systemic, chronic illness (the so-called clinical obesity), resulting in distinct clinical manifestations with specific signs/symptoms or limitations of activities of daily living. Pre clinical obesity confers an increased risk of developing clinical obesity as well as several other non-communicable diseases (NCDs), including T2D, CVD, certain types of cancer and mental illness. Because remission of clinical obesity does not imply cure, treatment of clinical obesity with resolution of clinical manifestations of organ dysfunction is matter of debate in order to evaluate the timing of the ongoing pharmacologic treatment. Clinical obesity may lead to severe organ dysfunction and end-organ damage, causing life-altering and/or potentially life-threatening complications. Coexisting obesity-related diseases should be considered in decision-making about indications to treatment and type of treatment.
The aim of this Collection is to provide a better understanding of the molecular and integrative mechanisms of the development of obesity and T2D, to ameliorate the clinical aspects and the relationships between both the diseases. A deeper knowledge of the specific profile and their multiple components may give a chance to a more efficient treatment in a personalized manner.
Possible topics include: basic mechanisms and clinal profiles of the patients, oxidative stress, chronic inflammation, hormonal and metabolic dysregulation, as areas involved in the development of obesity and/or T2D. Preventing strategies and newly developed agents to enhance fit behaviors and to contrast the pathogenetic mechanisms. Debate and research on advantages and disadvantages of measures of obesity and T2D and the outcomes after specific treatments.
Publishing Model: Hybrid
Deadline: Sep 30, 2026
Clinical and Translational Science in Pain and Headaches
Publishing Model: Hybrid
Deadline: Jun 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in