AI Reconstruction of the 12-Lead ECG via 3-Leads with Accurate Clinical Assessment
Published in Bioengineering & Biotechnology, Computational Sciences, and Biomedical Research

Myocardial Infarction (MI) affects millions of people each year and represents one of the major causes of death worldwide. Early detection is fundamental to prevent the most critical consequences of MI. Delayed diagnosis increases the risk of irreversible myocardial damage, and the rapid treatment of MI is thus vital for enhancing survival and long-term prognosis. According to standard clinical practices, MI diagnosis is achieved via a 12-lead Electrocardiogram (ECG), a clinical exam that monitors the heart's electrical activities via a complex set of 9 skin-surfaced electrodes. Specifically, six electrodes are placed on the patient's chest (precordial leads), while the other electrodes are placed on the patient's limb (limb leads). Because of the complexity of such a setup, 12-lead ECGs are performed only in clinical facilities with specific equipment, potentially delaying the detection of MIs.
In the last few years, technology development has enabled the recording of a subset of ECG leads via wearable devices or other agile solutions that can be used out-of-hospital. Therefore, we investigated if a subset of a few leads is sufficient to synthesize a full 12-lead ECG that can be used for clinical diagnosis. To this goal, we designed a neural network (NN) architecture and trained such a model over a dataset of more than 600'000 ECGs from more than 250'000 unique individuals. The dataset included various heart pathologies, including 18'000 signals presenting symptoms of acute MI.
We observed that two limb leads and one precordial lead are sufficient to reconstruct an accurate ECG. In particular, the most accurate reconstruction was obtained with lead V3, which is centrally placed within the precordial electrodes. To assess the accuracy of the reconstruction, we designed an algorithm that detects MI symptoms from full 12-lead ECGs. Hence, we tested the new algorithm on an ECG dataset including both original and reconstructed signals, assessing that there was no performance gap between the two configurations.
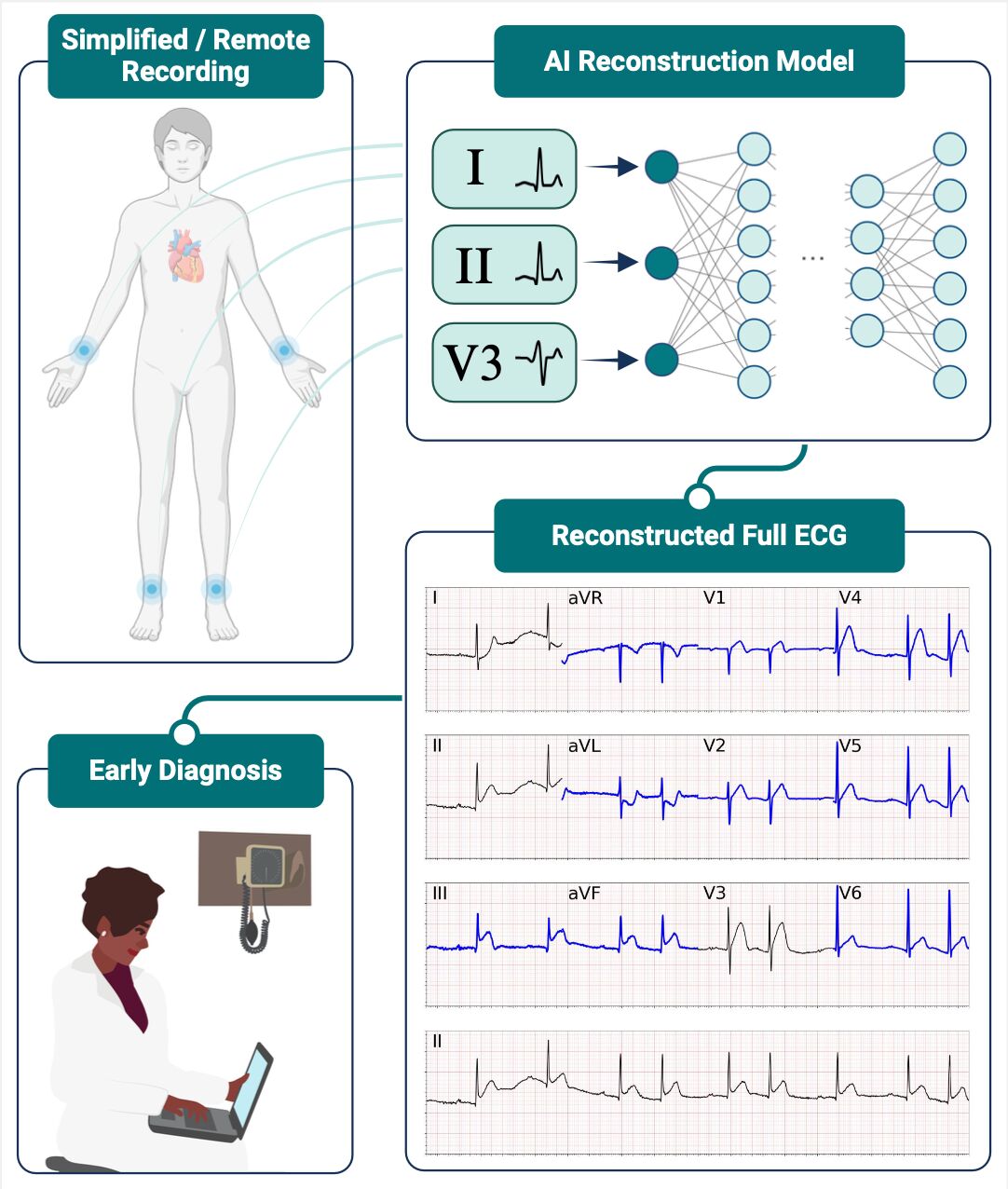
To prove the clinical utility of our method, we selected 238 ECGs, 119 presenting the signs of an ST-elevation MI (STEMI), and 119 controls. Each cardiologist was presented with one of the three distinct versions of 12-lead ECG: the original 12-lead ECG, the 12-lead ECG reconstructed from two limb leads only, and the 12-lead ECG reconstructed from limb leads and precordial lead. Each cardiologist was asked to determine which signals presented signs of STEMI, without knowing if the examined signal was an original or a synthetic ECG. In the end, reconstructed ECGs achieved an accuracy of 81.4 ± 5.0% in identifying ECG features of STE MI, comparable with the original 12-lead ECGs (accuracy 84.6 ± 4.6%). This result is very promising, showcasing that the reconstructed signal can be read correctly by a cardiologist.
Our solution may provide benefits in resource-limited care facilities or remote areas without adequate clinical infrastructure. In these environments, ECG reconstruction tools could aid in the early detection of STEMI and speed up medical intervention. Additionally, agile ECG recording systems could be useful in triage situations or other urgent settings with high patient volumes. Overall, our work represents a step towards direct-to-patient healthcare, potentially enabling home-based time-sensitive diagnoses, and ensuring that even marginalized and remote populations can receive timely and equitable care.
Follow the Topic
-
npj Digital Medicine

An online open-access journal dedicated to publishing research in all aspects of digital medicine, including the clinical application and implementation of digital and mobile technologies, virtual healthcare, and novel applications of artificial intelligence and informatics.
Your space to connect: The Primary immunodeficiency disorders Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine, Immunology, and Diseases!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Digital Health Equity and Access
Publishing Model: Open Access
Deadline: Mar 03, 2026
Evaluating the Real-World Clinical Performance of AI
Publishing Model: Open Access
Deadline: Jun 03, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Great Work