An aged maternal gut microbiome drives neuropsychiatric disorders in offspring. Targeting it could open new avenues for prevention
Published in Microbiology and Neuroscience

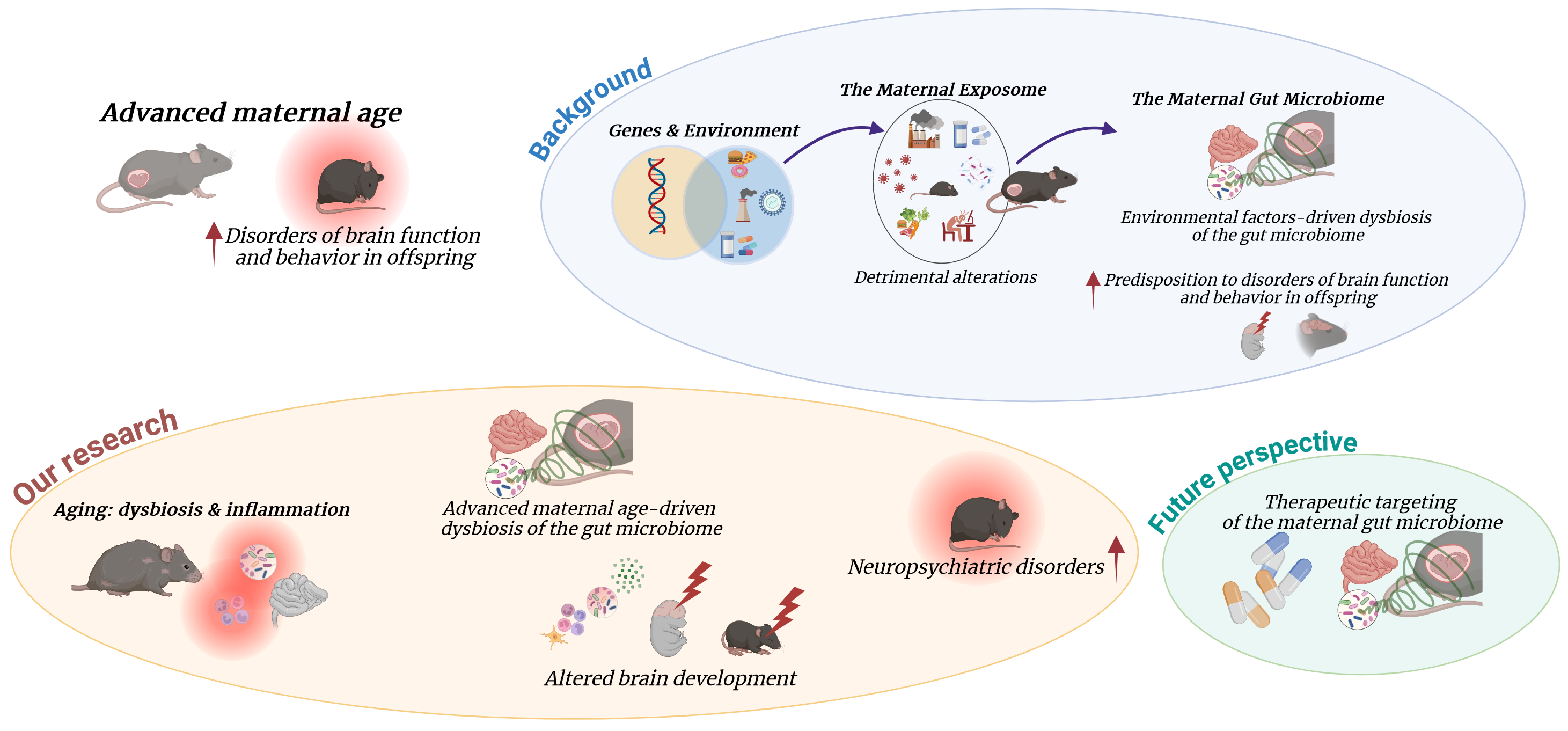
The average maternal age at childbirth is on the rise and affects the risk for neurodevelopmental and psychiatric disorders in offspring.
Driven by a range of economic, social, and medical factors, the proportion of women delaying childbirth is increasing globally. From 1990 to 2019, fertility rates for women aged 20 to 24 and 25 to 29 have dropped by 43% and 22%, respectively. Meanwhile, they have increased by 67% for women aged 35 to 39, and by over 132% for those aged 40 to 44. These trends are associated with an increased risk of a plethora of adverse maternal and neonatal outcomes. Additionally, advanced maternal age (AMA) at childbirth has been linked to higher rates of neuropsychiatric conditions in children and adults. However, the mechanisms by which AMA could potentially impact offspring neurodevelopment, and ultimately predispose to neuropsychiatric conditions later in life are still unclear. In our study, we aimed to explore the impact of factors associated with the aging process on early brain development and their influence on long-term behavioral outcomes. Our findings could open new avenues for preventive and treatment strategies to mitigate the incidence of neuropsychiatric disorders and their associated costs.
Maternal dysbiosis produces long-lasting behavioral changes in offspring
Project rationale and main findings
Developmental origins of health and disease: a role for age-related changes in the maternal gut microbiome?
A growing body of evidence supports the concept that chronic diseases have their origins in utero. Maternal environmental factors during pregnancy, such as metabolic disorders or infection, can impair both fetal and early post-natal development of the offspring, thus increasing the risk for chronic disorders in the offspring later in life. Research in the context of maternal obesity and infections has shown that effects may be mediated by the maternal gut microbiota, by intrauterine exposure to microbial metabolites involved in the immune and metabolic adaptations to pregnancy, and by postnatal mother-to-infant transmission of microbial species which contributes to the initial development of the neonatal microbiota.
In our previous work which led to this current study, we have found that aging correlates with gut dysbiosis and chronic low-grade inflammation, even in the absence of other risk factors and clinically active diseases. Despite the established association between aging, gut dysbiosis and increased inflammation, there is a scarcity of studies focusing on the effects of age-related maternal gut dysbiosis on fetal development and brain health outcomes. Intrigued by the possibility of a role for age-related alterations in the maternal gut microbiome in driving offspring neuropsychiatric outcomes, we hypothesized that in utero exposure to maternal gut dysbiosis and associated increased inflammation would be sufficient to drive behavioral alterations in offspring. Read the full paper here: Maternal dysbiosis produces long-lasting behavioral changes in offspring
Our research
To test our hypothesis, we needed to isolate the contribution of the maternal gut microbiome from other age-related maternal factors. To this end, we used a well-established approach in the field of microbiome research, the fecal microbiota transplantation (FMT), in a murine model. Gut communities from young vs aged donor females, which showed remarkable differences in terms of composition and structure, were transferred to young dams prior to mating. Intriguingly, the divergence in microbiome composition observed between young and aged donors was reflected in the recipient females after the transplantation. These age-related changes may influence the physiological remodeling of the maternal gut microbiome during pregnancy and hinder the immune and metabolic adaptations essential for fetal development. The first effect we observed was that colonizing young females with an aged microbiome significantly reduced fecundity and litter size, reflecting clinical findings that link AMA with increased infertility. Similar to findings in maternal obesity-related gut dysbiosis, the offspring of dams with an aged microbiome consistently displayed alterations in their gut microbiome compared to those of young microbiome dams, and these changes persisted into adulthood. These results highlighted that an aged maternal microbiome not only impacts the prenatal environment but can also influence brain development and function throughout the offspring's lifespan.
Since pathological alterations in the microbiome have been associated with behavioral changes in both human populations and pre-clinical models, we wanted to investigate whether maternal and offspring gut dysbiosis resulting from AMA could be sufficient to trigger behavioral dysfunction in the offspring. In line with our hypothesis, adult offspring from dams colonized with an aged microbiome displayed persistent depressive- and anxiety-like phenotypes, along with significant alterations in neurotransmitter levels and pro-inflammatory immune factors in both the blood and brain.
Thus, we provided evidence that age-related changes in the composition of the maternal gut microbiome contribute to chronic alterations in the behavior and physiology of offspring. As the first study to demonstrate a direct link between aging, antenatal gut dysbiosis, and the offspring's risk of neuropsychiatric disorders, our research has the potential to enhance understanding of how advanced maternal age impacts the brain health of descendants.
Implications for therapies and mitigating strategies
As microbiome research is rapidly progressing through advanced technologies to dissect the contribution of microbial factors to health and disease, the interest for microbiome-based therapeutics to treat and prevent brain disorders is growing across neuroscience and neurological research. In this context, our preclinical study not only contributes valuable insights into the mechanisms connecting maternal age and offspring brain health but also has significant translational implications, but also has significant translational implications for determining whether modifying an older maternal microbiome through prebiotic or probiotic interventions, or microbiota transfer therapies, can benefit both mothers and their offspring while potentially reducing the incidence of neuropsychiatric diseases in human populations.
Excited by these future perspectives, we have already begun to design and putting into action the next phases of this research to explore these promising avenues further.
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in