Antarctic haloarchaea – a unique Microbiome
Published in Microbiology

The paper in Microbiome is here: https://rdcu.be/1h8T
Haloarchaea are members of the domain Archaea that thrive in hypersaline environments. In Antarctica, the most well characterized haloarchaea come from a deep (36m), cold (as low as -20°C), marine-derived system called Deep Lake (DeMaere et al PNAS 2013). The lake is home to a low complexity community of microorganisms that is dominated by species of haloarchaea (Halohasta litchfieldiae [tADL], DL31 and Halorubrum lacusprofundi) that don’t tend to dominate in lower latitude systems. By environmental microbiology standards (where the majority of indigenous microbes are recalcitrant to laboratory isolation attempts), Hrr. lacusprofundi is exceptional in being amenable to isolation and cultivation. The ability to obtain new strains of the species have already proven to be of immense value with strain R1S1 providing the discovery of the plasmid (pR1SE) that ‘masquerades’ as a virus and disseminates via membrane vesicles (see Nature Research Microbiology Community); Erdmann et al Nat Microbiol 2017).
The Antarctic haloarchaea from Deep Lake have also been found to be quite ‘promiscuous’, capable of sharing long (up to 35kb), high identity (~100% nucleotide identity) regions (HIRs) of DNA (DeMaere et al PNAS 2013). The ability to do so has interesting implications for the ‘pan-genome’ of haloarchaeal species – i.e. the total pool of genetic material comprised by all members of a species (Tettelin et al PNAS 2005). Given the dominant Deep Lake Antarctic haloarchaeal species differ to those elsewhere in the world, questions arise as to the extent and uniqueness of the Antarctic haloarchaeal pan-genome.
In a study just published in Microbiome we set about defining differences between Hrr. lacusprofundi strain R1S1 (from Rauer 1 Lake) and strain ACAM34 (from Deep Lake). The strains provided the capacity to compare isolates from lakes located in different sectors of the East Antarctica region (one of 16 distinct ice-free biogeographic regions of Antarctica – Terrauds and Lee Diversity Distrib 2016): Rauer 1 Lake from Filla Island in the Rauer Island group, and Deep Lake ~ 30 km away and ~9 km ENE from Davis Station in the Vestfold Hills (image 1).
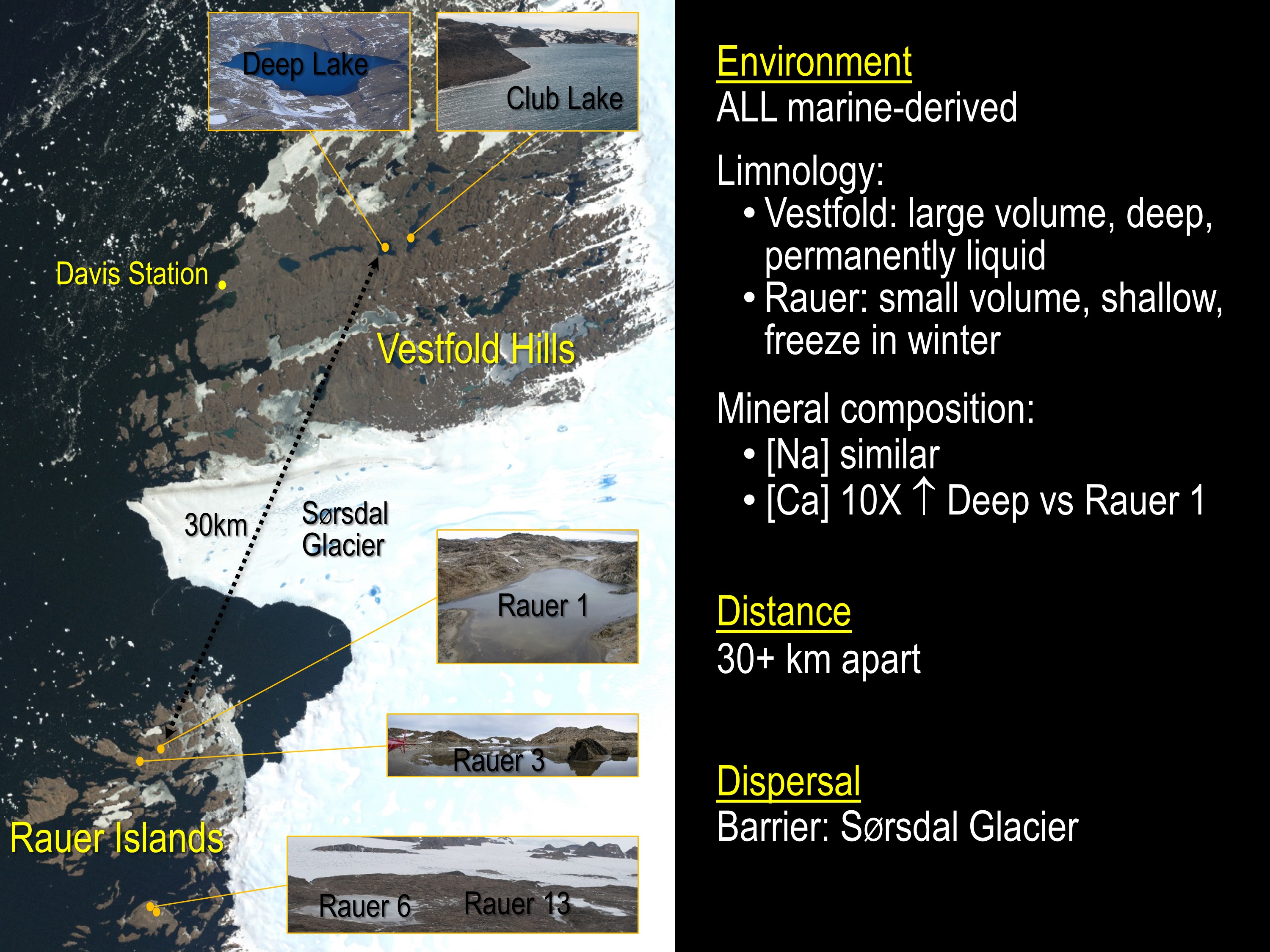
The analysis of strain variation formed the last chapter of Bernhard Tschitschko’s PhD studies. His analysis coincided with data beginning to churn out from our JGI Community Science Program that was (and still is) producing metagenome data from samples taken during a major 2013-2015 over-wintering expedition along with some others samples from 2006 and 2008. As a result, Bernhard and others (from my group, JGI and UTS) were able to analyse metagenome data generated from four hypersaline Rauer Island lakes (Rauer 1, 3, 6 and 13), Club Lake (which neighbours Deep Lake) and a Deep Lake time series (2006, 2008, 2013-2014). This enabled assessments of population level genomic variation including the prevalence of strain specific genomic markers, and biogeographic patterns of genome evolution.
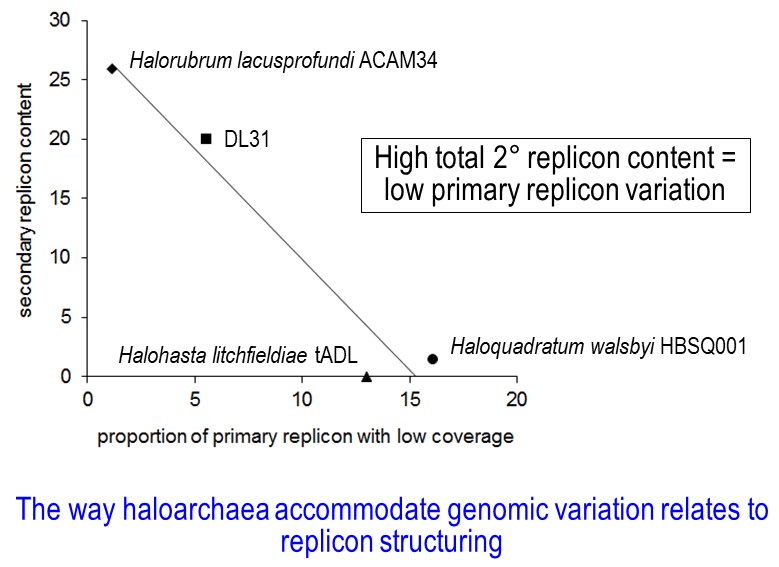
Because the work involved a very detailed analysis of genomic content and variation through to a comparative assessment of communities across disparate lake systems, the types of outcomes realized from the study were quite varied. For example, Bernhard found that the way that haloarchaea accommodate genomic variation relates to replicon structuring (image 2). Different species of haloarchaea have one replicon (chromosome) or multiple replicons (one large primary replicon and one or more secondary replicons). Bernhard determined that haloarchaea which have a high proportion of their genome contained in secondary replicons have a proportionately lower content of genomic variation in their primary replicon (and vice versa).
Consistent with Bernhard's previous study (Tschitschko et al ISMEJ 2015), he found that most of the Hrr. lacusprofundi strain variation could be associated with host-virus interactions (evasion/defence), with an interesting feature being the presence of an entire CRISPR/Cas region (~9.4 kb) representing a shared HIR with DL1 (a low abundance Halobacterium species). The study was also the first to use an Antarctic virus (isolated by Suzanne Erdmann) to perform infection studies on the two strains of Hrr. lacusprofundi, determining that one strain was easily infected and lysed, while the other strain was resistant.
In terms of grander implications, the study showed that specific haloarchaeal species were major species across the Rauer Islands and Vestfold Hills hypersaline lake systems (image 3).
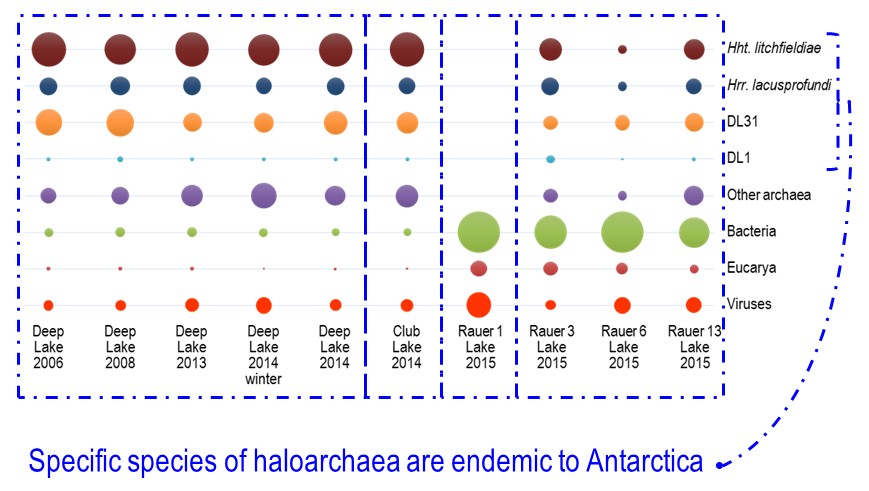
The dominant haloarchaeal populations possessed a high level of genomic conservation and also exhibited biogeographic distinctions (image 1 & 4).
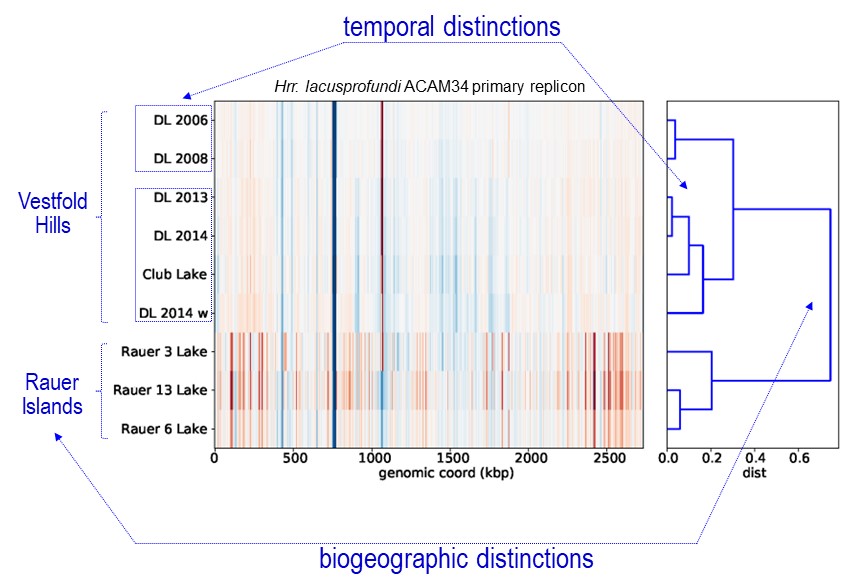
This indicates that these species are overall endemic to the Vestfold Hills-Rauer Islands and may very well be endemic to Antarctica. Very little is known about the endemism of Antarctic microorganisms (Cavicchioli Nat Rev Microbiol 2015): do regional distinctions occur (i.e. between the 16 distinct ice-free regions)?; does Antarctica have its own ‘total microbial pan-genome’ that varies to the rest of the world?; what does the extent of endemism mean for species invasion? – will the Antarctic populations be susceptible to alien species and hence ecosystem function become irrevocably perturbed? – one of our earlier studies suggests this could be the case for viral invasion of keystone bacterial species in Ace Lake (Lauro et al ISMEJ 2011).
Antarctic wildlife has some very real and profound issues to deal with. Antarctic waters are already experiencing pollution (plastics, drugs, wastes) and a high risk of invasive species and diseases due to several million people visiting per year (tourism, fisheries, research stations). In a focus issue of Nature on Antarctica, it was highlighted that Antarctica has in fact lost its ‘pristine’ status due to untoward human behaviour (Nature Editorial 2018). Hopefully it is studies like ours that will help to document just how much natural wonder exists and waits to be discovered in Antarctica – there are all the right reasons in the world to motivate sensible politics towards providing real and lasting protection for this unique, polar environment (Cavicchioli Greenpeace).
Article poster image photo credits: Alyce Hancock, Sarah Payne, Rick Cavicchioli
Follow the Topic
-
Microbiome

This journal hopes to integrate researchers with common scientific objectives across a broad cross-section of sub-disciplines within microbial ecology. It covers studies of microbiomes colonizing humans, animals, plants or the environment, both built and natural or manipulated, as in agriculture.
Related Collections
With Collections, you can get published faster and increase your visibility.
Harnessing plant microbiomes to improve performance and mechanistic understanding
This is a Cross-Journal Collection with Microbiome, Environmental Microbiome, npj Science of Plants, and npj Biofilms and Microbiomes. Please click here to see the collection page for npj Science of Plants and npj Biofilms and Microbiomes.
Modern agriculture needs to sustainably increase crop productivity while preserving ecosystem health. As soil degradation, climate variability, and diminishing input efficiency continue to threaten agricultural outputs, there is a pressing need to enhance plant performance through ecologically-sound strategies. In this context, plant-associated microbiomes represent a powerful, yet underexploited, resource to improve plant vigor, nutrient acquisition, stress resilience, and overall productivity.
The plant microbiome—comprising bacteria, fungi, and other microorganisms inhabiting the rhizosphere, endosphere, and phyllosphere—plays a fundamental role in shaping plant physiology and development. Increasing evidence demonstrates that beneficial microbes mediate key processes such as nutrient solubilization and uptake, hormonal regulation, photosynthetic efficiency, and systemic resistance to (a)biotic stresses. However, to fully harness these capabilities, a mechanistic understanding of the molecular dialogues and functional traits underpinning plant-microbe interactions is essential.
Recent advances in multi-omics technologies, synthetic biology, and high-throughput functional screening have accelerated our ability to dissect these interactions at molecular, cellular, and system levels. Yet, significant challenges remain in translating these mechanistic insights into robust microbiome-based applications for agriculture. Core knowledge gaps include identifying microbial functions that are conserved across environments and hosts, understanding the signaling networks and metabolic exchanges between partners, and predicting microbiome assembly and stability under field conditions.
This Research Topic welcomes Original Research, Reviews, Perspectives, and Meta-analyses that delve into the functional and mechanistic basis of plant-microbiome interactions. We are particularly interested in contributions that integrate molecular microbiology, systems biology, plant physiology, and computational modeling to unravel the mechanisms by which microbial communities enhance plant performance and/or mechanisms employed by plant hosts to assemble beneficial microbiomes. Studies ranging from controlled experimental systems to applied field trials are encouraged, especially those aiming to bridge the gap between fundamental understanding and translational outcomes such as microbial consortia, engineered strains, or microbiome-informed management practices.
Ultimately, this collection aims to advance our ability to rationally design and apply microbiome-based strategies by deepening our mechanistic insight into how plants select beneficial microbiomes and in turn how microbes shape plant health and productivity.
This collection is open for submissions from all authors on the condition that the manuscript falls within both the scope of the collection and the journal it is submitted to.
All submissions in this collection undergo the relevant journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief of the relevant journal. As an open access publication, participating journals levy an article processing fee (Microbiome, Environmental Microbiome). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief of the journal where the article is being submitted.
Collection policies for Microbiome and Environmental Microbiome:
Please refer to this page. Please only submit to one journal, but note authors have the option to transfer to another participating journal following the editors’ recommendation.
Collection policies for npj Science of Plants and npj Biofilms and Microbiomes:
Please refer to npj's Collection policies page for full details.
Publishing Model: Open Access
Deadline: Jun 01, 2026
Microbiome and Reproductive Health
Microbiome is calling for submissions to our Collection on Microbiome and Reproductive Health.
Our understanding of the intricate relationship between the microbiome and reproductive health holds profound translational implications for fertility, pregnancy, and reproductive disorders. To truly advance this field, it is essential to move beyond descriptive and associative studies and focus on mechanistic research that uncovers the functional underpinnings of the host–microbiome interface. Such studies can reveal how microbial communities influence reproductive physiology, including hormonal regulation, immune responses, and overall reproductive health.
Recent advances have highlighted the role of specific bacterial populations in both male and female fertility, as well as their impact on pregnancy outcomes. For example, the vaginal microbiome has been linked to preterm birth, while emerging evidence suggests that gut microbiota may modulate reproductive hormone levels. These insights underscore the need for research that explores how and why these microbial influences occur.
Looking ahead, the potential for breakthroughs is immense. Mechanistic studies have the power to drive the development of microbiome-based therapies that address infertility, improve pregnancy outcomes, and reduce the risk of reproductive diseases. Incorporating microbiome analysis into reproductive health assessments could transform clinical practice and, by deepening our understanding of host–microbiome mechanisms, lay the groundwork for personalized medicine in gynecology and obstetrics.
We invite researchers to contribute to this Special Collection on Microbiome and Reproductive Health. Submissions should emphasize functional and mechanistic insights into the host–microbiome relationship. Topics of interest include, but are not limited to:
- Microbiome and infertility
- Vaginal microbiome and pregnancy outcomes
- Gut microbiota and reproductive hormones
- Microbial influences on menstrual health
- Live biotherapeutics and reproductive health interventions
- Microbiome alterations as drivers of reproductive disorders
- Environmental factors shaping the microbiome
- Intergenerational microbiome transmission
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this collection undergo the journal’s standard peer review process. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jun 16, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in