Are macroscopic and microscopic properties always consistent with each in a small-charge world?
Published in Chemistry
Generally, the macroscopic and the microscopic behavior are consistent with each. For example, the microscopic friction is usually determined by measuring the macroscopic wetting property, such as the contact angle. Huang et al. have proposed that a quasi-universal relationship between the surface friction and the contact angles,(1) a large contact angle indicating a hydrophobic surface is associated with low surface friction, and vice versa. However, the macroscopic surface property is not always consistent with the microscopic. One example is the molecular-scale hydrophilicty at room temperature,(2-4) where a water droplet indicating the hydrophobic behavior at macroscopic scale coexists with a completely covered dense water monolayer indicating the microscopic hydrophilic behavior. This not only challenges the view that the macroscopic and the microscopic behavior is always consistent with each other, but also complicates the relationship between surface friction and surface hydrophobicity/hydrophilicity.(5)
The findings reported in our paper [https://doi.org/10.1038/s42004-020-0271-8] unexpectedly find, even over an order of magnitude difference of the friction coefficient at the small charge difference (≤0.36 e) on the two-dimensional material and biological lipid surfaces, despite of the similar contact angle values on these surfaces as traditionally thought. This large difference is confirmed by experimentally measuring surface friction of graphite and MoS2 using atomic force microscopy (AFM). The large variation of friction coefficient is attributed to the significant fluctuations of localized potential energy profile in presence of the inhomogeneous charge distributions. These results help clarifying the intrinsic physics relating macro wetting behaviors and micro frictions of various surfaces, including biological surfaces.
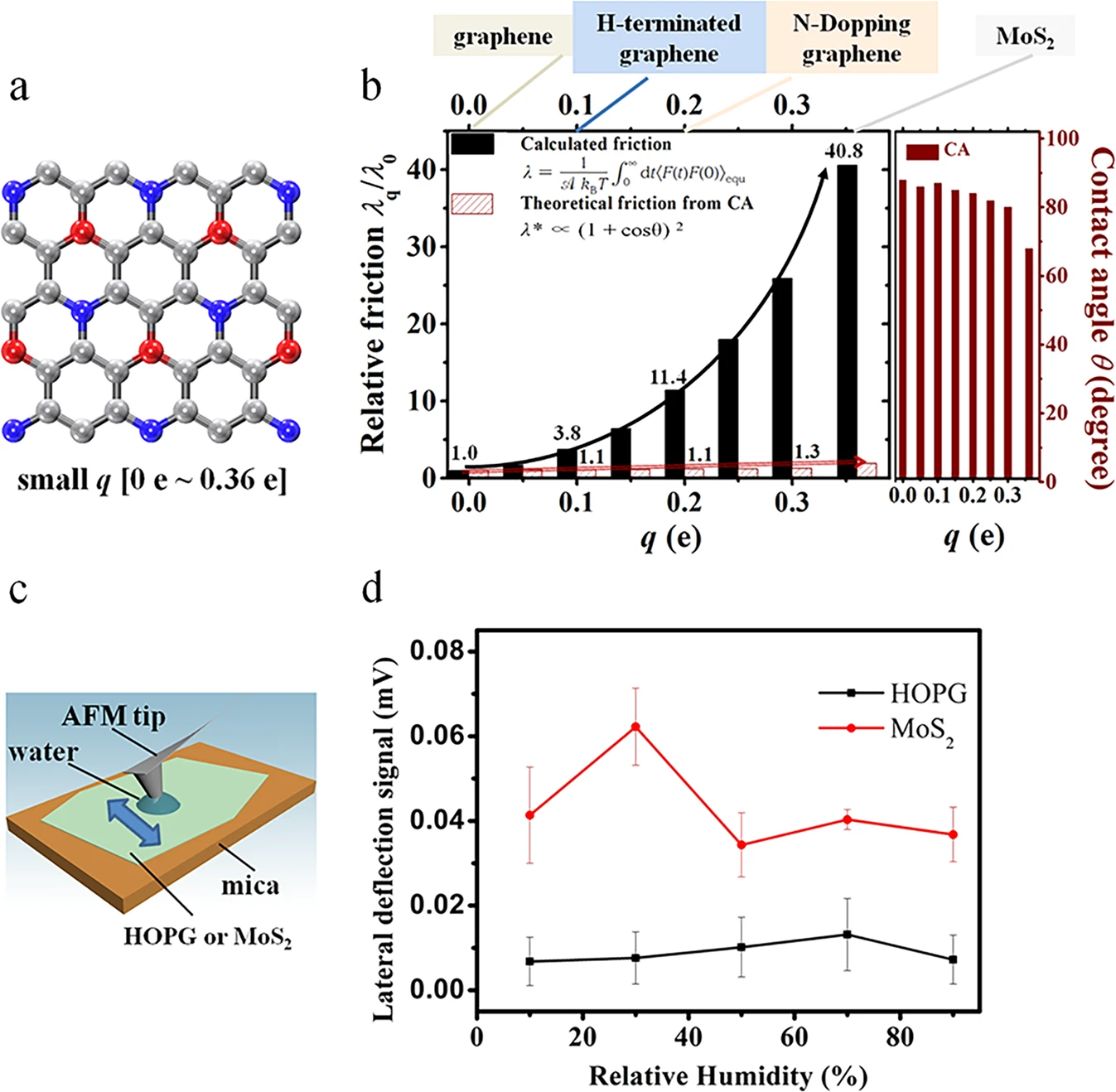
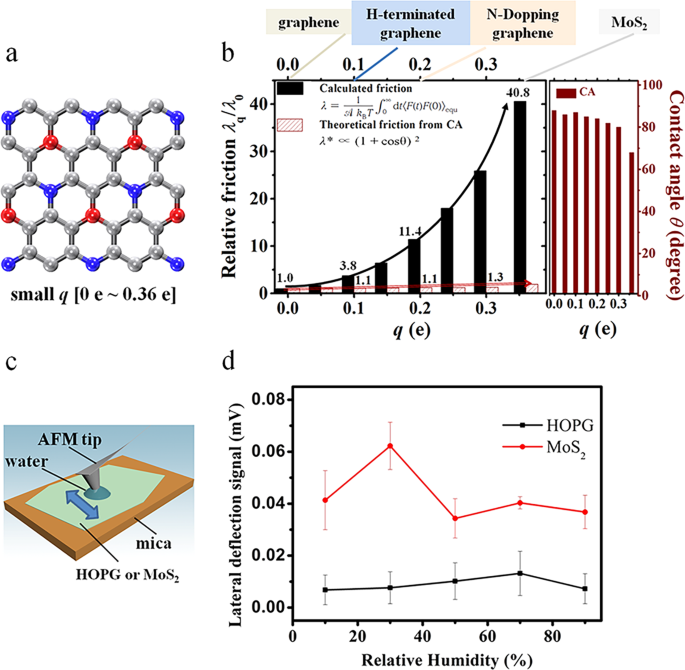
Figure 1. Friction coefficient for hexagonal solid surface, the highly oriented pyrolytic graphite (HOPG) and MoS2 solid surfaces. (a) Model hexagonal solid surface with small charge ranging from 0 e to 0.36 e. The red and blue spheres represent positive and negative charges, respectively, and the gray spheres represent neutral atoms. (b) Estimated relative friction coefficient λq/λ0 (q ≤ 0.36 e) of liquid water on the solid surface calculated with the Green-Kubo relationship (black solid columns), and relative friction (red textured columns) calculated from the contact angle using the theory proposed by Huang et al. (ref. 1), together with the contact angles in respect of the charge q values from 0.00 e to 0.36 e. When q increases from 0.00 e to 0.36 e, the Green-Kubo relative friction coefficient changes by a factor of 41 while the coefficient calculated from the contact angle changes by 50%. This panel also shows the typical materials with the atomic charge range from 0.00 e to 0.36 e, including graphene, hydrogen-terminated graphene, N-doped graphene, and MoS2. (c) Schematic for the measuring friction with an AFM tip. The arrow shows the moving direction of the AFM tip, which is perpendicular to the cantilever. A water droplet condenses from the humid environment between the hydrophilic AFM tip and the HOPG or MoS2. (d) Lateral deflection signal of the AFM cantilever when rubbing on HOPG or MoS2, respectively, varying with the relative environmental humidity. The friction force is proportional to the lateral deflection signal. Scale bars represent the standard deviation of the friction forces with the “discrete frequency” larger than 200.
Interestingly, we note that the most of popular two-dimensional materials and biomolecules (67.1% in all the residues atoms listed in OPLSAA force field) are formed by the atoms or groups with the small charges (≤ 0.36 e). For example, the carbon atoms in N-doped(6, 7) and the hydrogen terminated graphene(8) usually attain the charge from 0.10 e to 0.20 e, the carbon atoms of the terminal methyl of a lipid usually attain the charge of -0.18 e,(9-11) the carbon atoms of benzene rings in Phe group of protein residues can attain a charge of -0.115 e,(12, 13) and the S atoms of MoS2 have a charge of -0.36 e.(14). While, these small charges atoms/groups on the material and biomolecular surfaces are usually neglected in understanding the surface dynamics properties. This may be attributed to the negligible electrostatic interaction energy contribution of the total surface/water interactions due to the small charges.
For the materials with small charge, our results herein reveal that this general knowledge of wetting behaviors are not directly applied to infer the dynamics behaviors in respect of the frictions, even the wetting behavior, on the surfaces of the most popular two-dimensional materials(15) and biomolecules. Our findings not only open a door to design frictionless nano-devices, but also represent a big step towards understanding the microscopic nature of the small-charge world, such as the dynamics binding processes in protein-ligand in the crowded cell environments, molecular through the lipid membranes and the protein folding rates related to the internal frictions.
References
1. Huang DM, Sendner C, Horinek D, Netz RR, & Bocquet L (2008) Water Slippage versus Contact Angle: A Quasiuniversal Relationship. Phys. Rev. Lett. 101(22):226101.
2. Wang C, et al. (2009) Stable Liquid Water Droplet on a Water Monolayer Formed at Room Temperature on Ionic Model Substrates. Phys. Rev. Lett. 103:137801.
3. Shi G, et al. (2014) Molecular-scale Hydrophilicity Induced by Solute: Molecular-thick Charged Pancakes of Aqueous Salt Solution on Hydrophobic Carbon-based Surfaces. Sci. Rep. 4:6793.
4. Guo P, et al. (2015) Water-COOH Composite Structure with Enhanced Hydrophobicity Formed by Water Molecules Embedded into Carboxyl-Terminated Self-Assembled Monolayers. Phys. Rev. Lett. 115(18):186101.
5. Wang C, Wen B, Tu Y, Wan R, & Fang H (2015) Friction Reduction at a Superhydrophilic Surface: Role of Ordered Water. J. Phys. Chem. C 119:11679-11684.
6. Chaban VV & Prezhdo OV (2015) Nitrogen–Nitrogen Bonds Undermine Stability of N-Doped Graphene. J. Am. Chem. Soc. 137(36):11688-11694.
7. Chang J-K, et al. (2016) Spectroscopic studies of the physical origin of environmental aging effects on doped graphene. J. Appl. Phys. 119(23):235301.
8. Anithaa VS & Vijayakumar S (2018) Effect of side chain edge functionalization in pristine and defected graphene-DFT study. Computational and Theoretical Chemistry 1135:34-47.
9. von Hansen Y, Gekle S, & Netz RR (2013) Anomalous Anisotropic Diffusion Dynamics of Hydration Water at Lipid Membranes. Phys. Rev. Lett. 111(11):118103.
10. Schneck E, Sedlmeier F, & Netz RR (2012) Hydration repulsion between biomembranes results from an interplay of dehydration and depolarization. Proc. Natl. Acad. Sci. U. S. A 109(36):14405.
11. Tu Y, et al. (2013) Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nano. 8:968.
12. Tan P, et al. (2018) Gradual Crossover from Subdiffusion to Normal Diffusion: A Many-Body Effect in Protein Surface Water. Phys. Rev. Lett. 120(24):248101.
13. Shi G, et al. (2016) Unexpectedly Enhanced Solubility of Aromatic Amino Acids and Peptides in an Aqueous Solution of Divalent Transition-Metal Cations. Phys. Rev. Lett. 117(23):238102.
14. Luan B & Zhou R (2016) Wettability and friction of water on a MoS2 nanosheet. App. Phys. Lett. 108(13):131601.
15. Tocci G, Joly L, & Michaelides A (2014) Friction of Water on Graphene and Hexagonal Boron Nitride from Ab Initio Methods: Very Different Slippage Despite Very Similar Interface Structures. Nano Lett. 14(12):6872-6877.
Written by Chunlei Wang and Guosheng Shi.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in