Automation of customizable library preparation for next-generation sequencing into an open microfluidic platform
Published in Bioengineering & Biotechnology, Cancer, and Protocols & Methods

The challenge of automated library preparation for next-generation sequencing
In the rapidly developing landscape of cancer diagnostics, next-generation sequencing (NGS) has emerged as a transformative tool that enables the detection of mutations in e.g. cell-free DNA samples (cfDNA) that can serve as a basis for treatment decisions. However, the complexity of sequencing library preparation methods presents major challenges to roll-out NGS in a point-of-care setting. Factors including protocol complexity, contaminations, and costs are the three main challenges in preparing a sequencing library that can be overcome by automated systems [1]. Automation facilitates higher throughput, resulting in reduced costs and improved reproducibility, while also minimizing the risk of contamination [2].
Library preparation is a crucial step for the NGS procedure, where DNA fragments are prepared for sequencing. This process typically involves several steps, including fragmentation, end-repair, adapter ligation, and amplification. While these steps are essential for accurate sequencing, they are also time-consuming and error-prone, particularly when performed manually. Moreover, conventional liquid handling systems used in high-throughput laboratories are often too expensive and complex for smaller clinical settings. Furthermore, the device should be designed for use by non-experts, featuring a simple and flexible workflow that accommodates various library preparation methods to address several diagnostic approaches.
Given these challenges, there is a need for more streamlined, automated solutions that facilitate the use of NGS in routine clinical diagnostics, especially in smaller hospitals and medical offices where resources may be limited.
Automation of sequencing library preparation using lab-on-a-Chip technology
Within our recently published work, we used the commercially available Vivalytic cartridge from Bosch Healthcare Solutions GmbH (Waiblingen, Germany) to prepare a targeted sequencing library (Figure 1 a), which is open for different kinds of downstream diagnostic approaches. This approach integrates all necessary steps into a single, compact system and reduces the complexity contamination potential of manual workflows.
Therefore, we developed a multiplex PCR that targets multiple tumor-associated single nucleotide variants (SNVs) in a cell-free DNA (cfDNA) reference material. This biologically relevant reference material, which exhibits variable allelic frequencies, enables the assessment of the performance of the automated library preparation (referred to as 'on-chip') in comparison to the manual workflow (referred to as 'off-chip'). All necessary steps have been integrated into two cartridges to implement a sample preparation for NGS sequencing (Figure 1 b). These include all required enzyme reactions for targeted PCR, end-repair, ligation, and enrichment PCR. In addition, a DNA purification using carboxylated magnetic beads through solid-phase reversible immobilization was implemented. A separate quantification step was also integrated to convert the sample into a ready-to-load sequencing library.
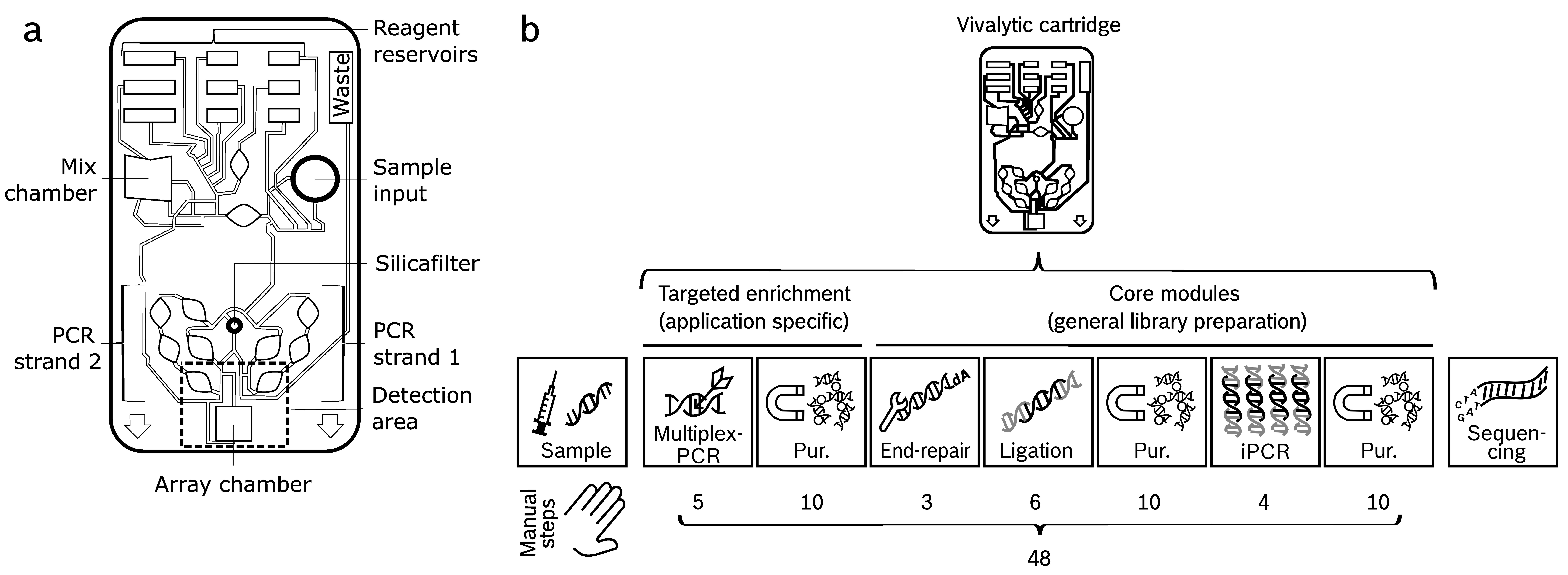
Figure 1: Schematic Vivalytic cartridge (a) and a workflow for a proof-of-concept for automated library preparation (b). a: Reagents can be pre-stored on the cartridge and transported via the microfluidic network to the different process units like the mixing chamber, PCR strands, or array chamber. The read-out of arrays and qPCRs can be analyzed with a camera system above the detection area. b: The library preparation requires 48 manual steps, which can be fully-automated on the Vivalytic cartridges. Pur.: Purification; iPCR: index PCR.
Evaluation of automated library preparation performance
For analyzing the performance of our automated library preparation we tested reference cfDNA with different mutational frequencies (0%, 0.1%/0.13%, 1%/1.3%, and 5%/6.3%). By comparing the results obtained from the automated system to those from a manual workflow, we could demonstrate a high level of correlation of 0.94 between all on- and off-chip libraries. This finding demonstrates that the automated platform effectively generates sequencing libraries that are comparable in quality to those prepared manually.
Conclusion and future directions
By simplifying the process of preparing samples for NGS by automation, the technology could make it feasible for smaller laboratories and clinics to adopt NGS as a diagnostic tool. This could be used in oncology, where timely detection of mutations can influence treatment decisions and patient outcomes.
In addition, the ability to perform NGS in a more accessible way could improve patient management. For example, liquid biopsies could become a regular routine test for monitoring tumor status and response to treatment, thus promoting personalized cancer treatment.
The described proof-of-concept on a microfluidic cartridge shows a successful preparation of targeted libraries with the potential to also preparing a universal whole genome sequencing (WGS) library by skipping the multiplex-PCR step. For use as a part of a diagnostic sequencing approach for small hospitals or ambulatory healthcare centers, real patient samples should be tested in future studies. Combined with further optimizations such as reducing the process to a single cartridge, the microfluidic library preparation could bring point-of-care sequencing forward.
References
[1] J. F. Hess et al., “Library preparation for next generation sequencing: A review of automation strategies,” Biotechnol. Adv., vol. 41, p. 107537, Jul. 2020, doi: 10.1016/j.biotechadv.2020.107537
[2] J. F. Hess et al., “Automation of Amplicon-Based Library Preparation for Next-Generation Sequencing by Centrifugal Microfluidics,” Anal. Chem., vol. 92, no. 19, pp. 12833–12841, Aug. 2020, doi: 10.1021/acs.analchem.0c01202
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in