BA.5 infection results in milder pathology than ancestral SARS-CoV-2 Wuhan strain infection
Published in Microbiology
Background
Viruses undergo mutations that may increase their virulence making them more pathogenic. Mutations in severe acquired respiratory syndrome coronavirus-2 (SARS-CoV-2) the causative agent for coronavirus disease have led to the emergence of SARS-CoV-2 variants with increased virulence and compromised protection in COVID-19 vaccinated individuals. While initial variants, such as the Delta strain were reported to be more pathogenic resulting in increased cases of morbidity, hospitalization, and mortality. The Omicron variant and Omicron sub-variants such as BA.1, BA.2, BA.3, and BA.4 were reported to be less pathogenic as compared to ancestral SARS-CoV-2 strain (Wuhan-Hu-1) but led to fourth epidemic wave in South Africa due to high transmissibility. However, not much is known about the pathology of recently emerged BA.5 infection necessitating the investigation of BA.5 infection pathology and immune response by using animal models.
Unanswered questions
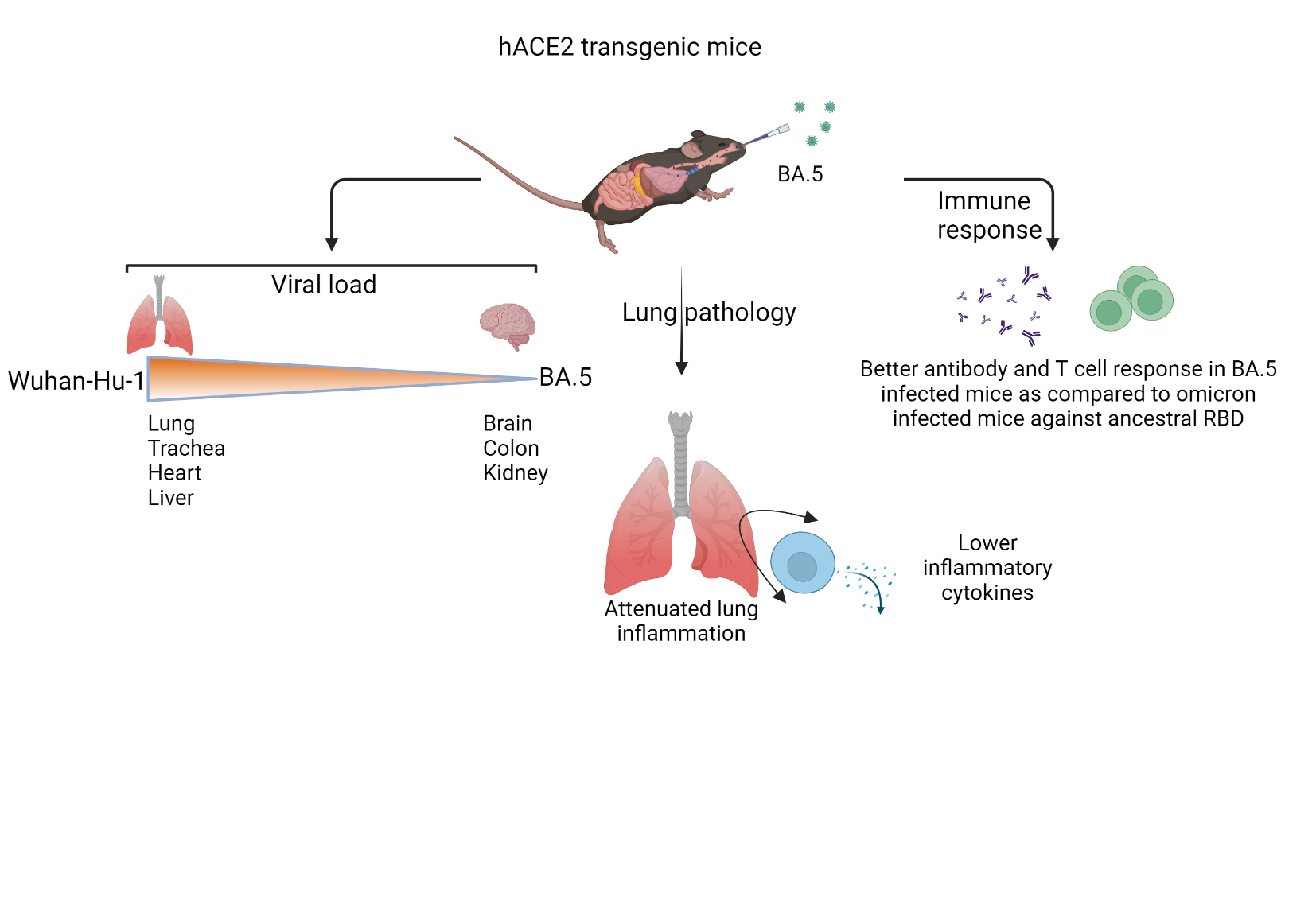
In the current study, we used hACE2 transgenic mice to understand the pathology of BA.5 infections. These mice have human ACE2 receptors which allows SARS-CoV-2 infection in these mice to mimic human pathology. By using these mice, we asked what the severity of BA.5 infections as compared to Wuhan-Hu-1 or Omicron strain in terms of the number of viruses present in the lungs as well as the degree of lung pathology after infection. We also investigated the ability of BA.5 to infect other vital organs and the immune response. Finally, we also tried to understand the pathology of BA.5 infections in omicron-recovered animals which is important for evaluating
Our findings
These mice express human ACE2 receptor which supports virus entry and replication in the lungs of infected mice. The infected hACE2 transgenic mice show acute lethal pathology when infected with SARS-CoV-2 ancestral Wuhan-Hu-1, mimicking severe cases of COVID-19. When BA.5 was infected in these mice, there was no mortality observed and the lungs of the infected mice showed milder pathological symptoms suggesting lower severity. In addition, the inflammatory cytokine in the lungs that is responsible for lung injury was also lower in case of BA.5 infection. Interestingly, the viral load in other vital organs such as Brain, Colon, and Kidney were higher in BA.5 infection as compared to Wuhan-Hu-1 infection. Another important outcome of the study was that the mice infected previously with Omicron and re-infected with BA.5 did not show any pathological signs. Suggesting that the individuals previously exposed to Omicron remain protected against BA.5 infections. Taken together, our study provides immunopathological findings that would be helpful in understanding the BA.5 infection and immunological response.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in