Bacterial dilemma: To float or not to float
Published in Microbiology

Explore the Research

Systems view of Bacillus subtilis pellicle development - npj Biofilms and Microbiomes
npj Biofilms and Microbiomes - Systems view of Bacillus subtilis pellicle development
Life is made up of many dilemmas and decisions: to study or not to study, what to study and what to exclude, which methods to use or not to use and many, many more that determine the outcome of the study. It appears that even the simplest creatures are not exempt from making decisions. The bacterial decision to make a biofilm at the water-air interface or the water-solid interface is one of such dilemmas which, however, comes with a dire consequence.
Given the past research in our lab the decision on what to study was quite simple - we will study biofilms of the Gram positive bacteria Bacillus subtilis. It is one of the best-studied microbial biofilm model systems and can form biofilms on almost any surface. We intend to study biofilms at the water-air interface and not the other forms of biofilms. Biofilms at the water-air interface are typically observed in unshaken growth systems, can be easily removed from the system, and studied with various dedicated techniques. But removing the biofilm from the growth system may easily destroy its delicate structure which is frustrating if one wants to study its structure. Studying biofilms at the water-air interface is exciting as it depends on the dynamics of both water and atmosphere phase to which it borders. Changes in the atmosphere can be mostly neglected, but the same cannot be said for the water phase where bacteria are growing, and would continue to grow, if there would not be the deteriorating conditions created by growth.
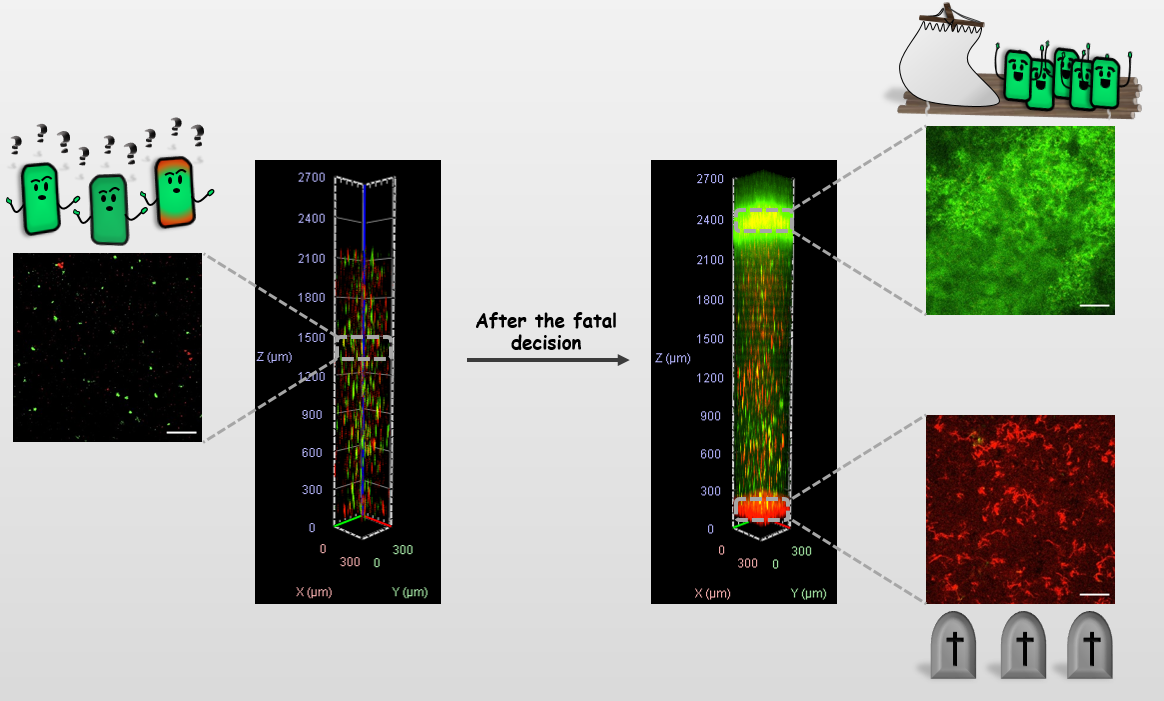
Furthermore, the real challenge of this study, was which methods to use to couple the growth of bacteria in the suspension with the biofilm formation at the water-air interface. It turned up that the majority of bacteria that were in the suspension did not make it to the interface, but died during the process. We have applied rotational biconical interfacial rheology measuring systems for in situ and real-time measurement to monitor biofilm structure formation at the interface. But this didn’t give us the answer to what was going on in the suspension prior to the biofilm formation. The idea came from two undergraduate students in the lab who were working on the visualization of the vertical distribution of bacteria in a relatively large water column. They managed to scan the bacterial distribution in the water column at over 2000 mm of depth. Therefore, we have optimized their method to continuously scan the bacterial distribution in the water column before and during biofilm development, and through its collapse which allowed us to obtain a systems view on biofilm formation.
The results portrayed a new picture of bacterial redistribution prior to the formation of the biofilm at the water-air interface. After inoculation bacteria were uniformly distributed and viable through the water column. However, the crowding of the bacterial cells deteriorated the growth conditions and the bacteria had to make the decision that sealed their fate. Bacteria could choose between water suspension, solid-water, and water-air interface. By monitoring bacteria in the entire water column we noticed that only bacteria that swim to the air-water interface made the right decision. At the water-air interface with access to both oxygen and nutrients bacteria formed micro aggregates which entangled in bacterial filaments and after approximately 15 h formed confluent and robust biofilm. The bacteria that decided to stay in the suspension were eventually deprived of energy and sank to the bottom of the water column where they form a sedimented biofilm, much thinner than the air-water biofilm, that was composed of bacteria with severely compromised membranes and was equivalent to a pile of dead bacteria. The decision to stay in the suspension was deadly and akin to committing suicide.
A holistic view of the system suggests that biofilm formation at the water-air interface is a rescue mission for a dying population. A reduction of the viable habitat to the water-air interface forces cell development, morphogenesis, and build-up of mechanical stress supporting structure that can be seen as building a small inflatable life raft that enables survival of a subpopulation of submariners in the need of oxygen. The life raft, however, can take up only a limited number of passengers before it capsizes. An increasing height of the growing raft reduces nutrient availability and results in a finite pellicle thickness. Eventually, the conditions on the upper deck of the life raft become intolerable and bacteria produce spores in a final rescue operation to ensure survival. However, the release of enzymes during spore formation deflates the life raft and its ability to support mechanical stress is lost. The biofilm disintegrates, sinks to the bottom, and joins the rest of the sunken crew. And the system now enters into dormancy. What a spectacular journey for bacteria and a PhD student with some right decisions along the way.
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: May 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Nice story and diagram!
Thanks :)!