Behind the Paper: Enhancing Parkinson's Disease Monitoring with Digital Gait Data and Multimodal Data Integration
Published in Chemistry, Electrical & Electronic Engineering, and Neuroscience
What Did the Researchers Discover?
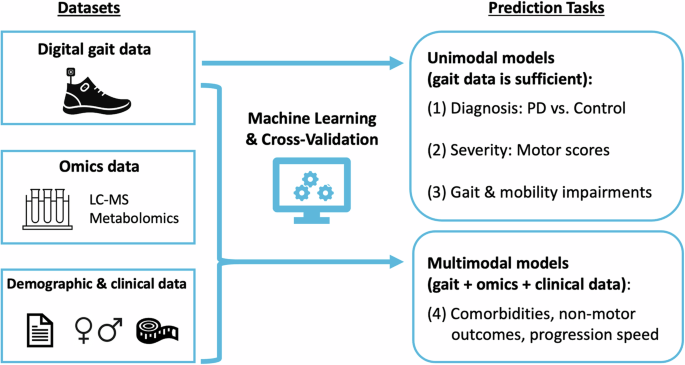
The researchers explored the use of digital gait data, along with metabolomics and clinical data, to predict various health outcomes in Parkinson's disease (PD). They found that integrating these different types of data can help to improve the ability to monitor and diagnose specific impairments in PD patients. Specifically, this multimodal approach improved the prediction of complex outcomes, such as cognitive impairment and hallucinations, which are difficult to detect using a single type of data. Moreover, gait-based machine learning models used in the study demonstrated significant accuracy, with AUC scores ranging from 83-92% for discriminating PD patients from controls and up to 75% for predicting motor severity.
How Do Digital Biomarkers Help in Parkinson’s Disease?
IImagine a PD patient wearing a special pair of shoes equipped with digital sensors that continuously monitor his or her gait. This seamlessly collected, objective measurement data can reveal subtle motor impairments that might not be easily detected during a standard clinical visit. When this gait data is combined with metabolomic markers (biochemicals in the blood) and clinical information, it provides a more comprehensive view of the disease. For example, digital gait markers alone were effective in predicting motor impairment and distinguishing Parkinson's patients from healthy individuals. However, for non-motor symptoms such as cognitive decline or hallucinations, integrating gait data with metabolomics and clinical data significantly improved prediction accuracy.
Why Is This Important?
Understanding how to integrate different types of data to more effectively monitor Parkinson's disease is important to advancing personalized medicine. Traditional clinical assessments can be time-consuming and may miss subtle changes in a patient's condition. Digital biomarkers provide a non-invasive, continuous and objective way to monitor disease progression. When combined with metabolomics and clinical data, these markers can improve the ability to predict difficult-to-detect outcomes, paving the way for more personalized treatment plans.
What Were the Challenges They Faced During This Research?
Collecting and integrating high-quality data from multiple modalities presented several challenges. Ensuring the accuracy, reliability, and informative value of digital gait data required specialized data processing procedures. In addition, the collection of metabolomics data, which involves complex biochemical analyses, demanded a well-matched study design to minimize variability. Building machine learning models that could effectively combine these different types of data also required careful tuning to avoid overfitting and ensure generalizability. In addition, the multimodal analysis of three different data types (digital gait data, metabolomics data, and clinical data) presented unique challenges in terms of data integration, feature selection, and interpretation.
Any Surprising Findings?
A surprising finding from the study was the identification of specific metabolites that had not previously been associated with Parkinson's disease, but showed strong predictive power for certain symptoms in our combined study with digital gait data. For example, the metabolite 3-hydroxy-2-ethylpropionate was a top predictor of dopamine dysregulation syndrome, a challenging comorbidity in PD. Similarly, other metabolites such as gamma-glutamylvaline, which is involved in glutathione metabolism, emerged as significant predictors of depression outcomes. Another unexpected finding was that the integration of clinical, metabolomic, and gait data led to significant improvements in predicting outcomes such as hallucinations and dyskinesia compared to using either type of data alone. This highlights the value of combining different types of data to capture the complex and multifaceted nature of Parkinson's disease. These surprising findings open new avenues for research into the biological basis of non-motor symptoms in PD and suggest that multimodal data integration can reveal insights that would not be apparent using a single data modality.
What's Next?
While the study showed promising results, it also had limitations, such as being conducted at a single center and using a cross-sectional design. Future research should aim to include more diverse patient populations and use longitudinal designs to better understand how these digital and biochemical markers change over time in relation to disease progression. In addition, exploring the integration of other types of data, such as neuroimaging, could further improve the predictive models and facilitate the development of more personalized management strategies for PD.
Follow the Topic
-
npj Digital Medicine

An online open-access journal dedicated to publishing research in all aspects of digital medicine, including the clinical application and implementation of digital and mobile technologies, virtual healthcare, and novel applications of artificial intelligence and informatics.
Related Collections
With Collections, you can get published faster and increase your visibility.
Evaluating the Real-World Clinical Performance of AI
Publishing Model: Open Access
Deadline: Jun 03, 2026
Impact of Agentic AI on Care Delivery
Publishing Model: Open Access
Deadline: Jul 12, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in